Abstract
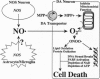
1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) causes nigrostriatal dopaminergic pathway damage similar to that observed in Parkinson disease (PD). To study the role of NO radical in MPTP-induced neurotoxicity, we injected MPTP into mice in which nitric oxide synthase (NOS) was inhibited by 7-nitroindazole (7-NI) in a time- and dose-dependent fashion. 7-NI dramatically protected MPTP-injected mice against indices of severe injury to the nigrostriatal dopaminergic pathway, including reduction in striatal dopamine contents, decreases in numbers of nigral tyrosine hydroxylase-positive neurons, and numerous silver-stained degenerating nigral neurons. The resistance of 7-NI-injected mice to MPTP is not due to alterations in striatal pharmacokinetics or content of 1-methyl-4-phenylpyridinium ion (MPP+), the active metabolite of MPTP. To study specifically the role of neuronal NOS (nNOS), MPTP was administered to mutant mice lacking the nNOS gene. Mutant mice are significantly more resistant to MPTP-induced neurotoxicity compared with wild-type littermates. These results indicate that neuronally derived NO mediates, in part, MPTP-induced neurotoxicity. The similarity between the MPTP model and PD raises the possibility that NO may play a significant role in the etiology of PD.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beckman J. S., Beckman T. W., Chen J., Marshall P. A., Freeman B. A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman J. S., Chen J., Crow J. P., Ye Y. Z. Reactions of nitric oxide, superoxide and peroxynitrite with superoxide dismutase in neurodegeneration. Prog Brain Res. 1994;103:371–380. doi: 10.1016/s0079-6123(08)61151-6. [DOI] [PubMed] [Google Scholar]
- Beckman J. S., Ischiropoulos H., Zhu L., van der Woerd M., Smith C., Chen J., Harrison J., Martin J. C., Tsai M. Kinetics of superoxide dismutase- and iron-catalyzed nitration of phenolics by peroxynitrite. Arch Biochem Biophys. 1992 Nov 1;298(2):438–445. doi: 10.1016/0003-9861(92)90432-v. [DOI] [PubMed] [Google Scholar]
- Beckman J. S. Peroxynitrite versus hydroxyl radical: the role of nitric oxide in superoxide-dependent cerebral injury. Ann N Y Acad Sci. 1994 Nov 17;738:69–75. doi: 10.1111/j.1749-6632.1994.tb21791.x. [DOI] [PubMed] [Google Scholar]
- Beckman J. S. The double-edged role of nitric oxide in brain function and superoxide-mediated injury. J Dev Physiol. 1991 Jan;15(1):53–59. [PubMed] [Google Scholar]
- Berger N. A. Poly(ADP-ribose) in the cellular response to DNA damage. Radiat Res. 1985 Jan;101(1):4–15. [PubMed] [Google Scholar]
- Bland-Ward P. A., Moore P. K. 7-Nitro indazole derivatives are potent inhibitors of brain, endothelium and inducible isoforms of nitric oxide synthase. Life Sci. 1995;57(11):PL131–PL135. doi: 10.1016/0024-3205(95)02046-l. [DOI] [PubMed] [Google Scholar]
- Bredt D. S., Glatt C. E., Hwang P. M., Fotuhi M., Dawson T. M., Snyder S. H. Nitric oxide synthase protein and mRNA are discretely localized in neuronal populations of the mammalian CNS together with NADPH diaphorase. Neuron. 1991 Oct;7(4):615–624. doi: 10.1016/0896-6273(91)90374-9. [DOI] [PubMed] [Google Scholar]
- Dawson T. M., Snyder S. H. Gases as biological messengers: nitric oxide and carbon monoxide in the brain. J Neurosci. 1994 Sep;14(9):5147–5159. doi: 10.1523/JNEUROSCI.14-09-05147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun N. J., Dun S. L., Förstermann U. Nitric oxide synthase immunoreactivity in rat pontine medullary neurons. Neuroscience. 1994 Mar;59(2):429–445. doi: 10.1016/0306-4522(94)90607-6. [DOI] [PubMed] [Google Scholar]
- Dun N. J., Dun S. L., Wu S. Y., Förstermann U., Schmidt H. H., Tseng L. F. Nitric oxide synthase immunoreactivity in the rat, mouse, cat and squirrel monkey spinal cord. Neuroscience. 1993 Jun;54(4):845–857. doi: 10.1016/0306-4522(93)90579-5. [DOI] [PubMed] [Google Scholar]
- Forno L. S., DeLanney L. E., Irwin I., Langston J. W. Similarities and differences between MPTP-induced parkinsonsim and Parkinson's disease. Neuropathologic considerations. Adv Neurol. 1993;60:600–608. [PubMed] [Google Scholar]
- Francis J. W., Von Visger J., Markelonis G. J., Oh T. H. Neuroglial responses to the dopaminergic neurotoxicant 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in mouse striatum. Neurotoxicol Teratol. 1995 Jan-Feb;17(1):7–12. doi: 10.1016/0892-0362(94)00048-i. [DOI] [PubMed] [Google Scholar]
- Fuller R. W., Hahn R. A., Snoddy H. D., Wikel J. H. Depletion of cardiac norepinephrine in rats and mice by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Biochem Pharmacol. 1984 Oct 1;33(19):2957–2960. doi: 10.1016/0006-2952(84)90593-8. [DOI] [PubMed] [Google Scholar]
- Gallyas F., Wolff J. R., Böttcher H., Zaborszky L. A reliable and sensitive method to localize terminal degeneration and lysosomes in the central nervous system. Stain Technol. 1980 Sep;55(5):299–306. doi: 10.3109/10520298009067258. [DOI] [PubMed] [Google Scholar]
- Giovanni A., Sieber B. A., Heikkila R. E., Sonsalla P. K. Correlation between the neostriatal content of the 1-methyl-4-phenylpyridinium species and dopaminergic neurotoxicity following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine administration to several strains of mice. J Pharmacol Exp Ther. 1991 May;257(2):691–697. [PubMed] [Google Scholar]
- Hallman H., Lange J., Olson L., Strömberg I., Jonsson G. Neurochemical and histochemical characterization of neurotoxic effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine on brain catecholamine neurones in the mouse. J Neurochem. 1985 Jan;44(1):117–127. doi: 10.1111/j.1471-4159.1985.tb07120.x. [DOI] [PubMed] [Google Scholar]
- Hallman H., Olson L., Jonsson G. Neurotoxicity of the meperidine analogue N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine on brain catecholamine neurons in the mouse. Eur J Pharmacol. 1984 Jan 13;97(1-2):133–136. doi: 10.1016/0014-2999(84)90521-1. [DOI] [PubMed] [Google Scholar]
- Hasegawa E., Takeshige K., Oishi T., Murai Y., Minakami S. 1-Methyl-4-phenylpyridinium (MPP+) induces NADH-dependent superoxide formation and enhances NADH-dependent lipid peroxidation in bovine heart submitochondrial particles. Biochem Biophys Res Commun. 1990 Aug 16;170(3):1049–1055. doi: 10.1016/0006-291x(90)90498-c. [DOI] [PubMed] [Google Scholar]
- Heikkila R. E., Hess A., Duvoisin R. C. Dopaminergic neurotoxicity of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine in mice. Science. 1984 Jun 29;224(4656):1451–1453. doi: 10.1126/science.6610213. [DOI] [PubMed] [Google Scholar]
- Heikkila R. E., Manzino L., Cabbat F. S., Duvoisin R. C. Protection against the dopaminergic neurotoxicity of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine by monoamine oxidase inhibitors. Nature. 1984 Oct 4;311(5985):467–469. doi: 10.1038/311467a0. [DOI] [PubMed] [Google Scholar]
- Heikkila R. E., Sieber B. A., Manzino L., Sonsalla P. K. Some features of the nigrostriatal dopaminergic neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) in the mouse. Mol Chem Neuropathol. 1989 Jun;10(3):171–183. doi: 10.1007/BF03159727. [DOI] [PubMed] [Google Scholar]
- Herkenham M., Little M. D., Bankiewicz K., Yang S. C., Markey S. P., Johannessen J. N. Selective retention of MPP+ within the monoaminergic systems of the primate brain following MPTP administration: an in vivo autoradiographic study. Neuroscience. 1991;40(1):133–158. doi: 10.1016/0306-4522(91)90180-v. [DOI] [PubMed] [Google Scholar]
- Huang P. L., Dawson T. M., Bredt D. S., Snyder S. H., Fishman M. C. Targeted disruption of the neuronal nitric oxide synthase gene. Cell. 1993 Dec 31;75(7):1273–1286. doi: 10.1016/0092-8674(93)90615-w. [DOI] [PubMed] [Google Scholar]
- Jackson-Lewis V., Jakowec M., Burke R. E., Przedborski S. Time course and morphology of dopaminergic neuronal death caused by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurodegeneration. 1995 Sep;4(3):257–269. doi: 10.1016/1055-8330(95)90015-2. [DOI] [PubMed] [Google Scholar]
- Javitch J. A., D'Amato R. J., Strittmatter S. M., Snyder S. H. Parkinsonism-inducing neurotoxin, N-methyl-4-phenyl-1,2,3,6 -tetrahydropyridine: uptake of the metabolite N-methyl-4-phenylpyridine by dopamine neurons explains selective toxicity. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2173–2177. doi: 10.1073/pnas.82.7.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenner P., Schapira A. H., Marsden C. D. New insights into the cause of Parkinson's disease. Neurology. 1992 Dec;42(12):2241–2250. doi: 10.1212/wnl.42.12.2241. [DOI] [PubMed] [Google Scholar]
- Kelly P. A., Ritchie I. M., Arbuthnott G. W. Inhibition of neuronal nitric oxide synthase by 7-nitroindazole: effects upon local cerebral blood flow and glucose use in the rat. J Cereb Blood Flow Metab. 1995 Sep;15(5):766–773. doi: 10.1038/jcbfm.1995.96. [DOI] [PubMed] [Google Scholar]
- Kopin I. J., Markey S. P. MPTP toxicity: implications for research in Parkinson's disease. Annu Rev Neurosci. 1988;11:81–96. doi: 10.1146/annurev.ne.11.030188.000501. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langston J. W., Irwin I. MPTP: current concepts and controversies. Clin Neuropharmacol. 1986;9(6):485–507. [PubMed] [Google Scholar]
- Markey S. P., Johannessen J. N., Chiueh C. C., Burns R. S., Herkenham M. A. Intraneuronal generation of a pyridinium metabolite may cause drug-induced parkinsonism. Nature. 1984 Oct 4;311(5985):464–467. doi: 10.1038/311464a0. [DOI] [PubMed] [Google Scholar]
- Moore P. K., Wallace P., Gaffen Z., Hart S. L., Babbedge R. C. Characterization of the novel nitric oxide synthase inhibitor 7-nitro indazole and related indazoles: antinociceptive and cardiovascular effects. Br J Pharmacol. 1993 Sep;110(1):219–224. doi: 10.1111/j.1476-5381.1993.tb13795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthane U., Ramsay K. A., Jiang H., Jackson-Lewis V., Donaldson D., Fernando S., Ferreira M., Przedborski S. Differences in nigral neuron number and sensitivity to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in C57/bl and CD-1 mice. Exp Neurol. 1994 Apr;126(2):195–204. doi: 10.1006/exnr.1994.1058. [DOI] [PubMed] [Google Scholar]
- Nicklas W. J., Youngster S. K., Kindt M. V., Heikkila R. E. MPTP, MPP+ and mitochondrial function. Life Sci. 1987 Feb 23;40(8):721–729. doi: 10.1016/0024-3205(87)90299-2. [DOI] [PubMed] [Google Scholar]
- O'Dell T. J., Huang P. L., Dawson T. M., Dinerman J. L., Snyder S. H., Kandel E. R., Fishman M. C. Endothelial NOS and the blockade of LTP by NOS inhibitors in mice lacking neuronal NOS. Science. 1994 Jul 22;265(5171):542–546. doi: 10.1126/science.7518615. [DOI] [PubMed] [Google Scholar]
- Przedborski S., Jackson-Lewis V., Kostic V., Carlson E., Epstein C. J., Cadet J. L. Superoxide dismutase, catalase, and glutathione peroxidase activities in copper/zinc-superoxide dismutase transgenic mice. J Neurochem. 1992 May;58(5):1760–1767. doi: 10.1111/j.1471-4159.1992.tb10051.x. [DOI] [PubMed] [Google Scholar]
- Przedborski S., Kostic V., Jackson-Lewis V., Naini A. B., Simonetti S., Fahn S., Carlson E., Epstein C. J., Cadet J. L. Transgenic mice with increased Cu/Zn-superoxide dismutase activity are resistant to N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity. J Neurosci. 1992 May;12(5):1658–1667. doi: 10.1523/JNEUROSCI.12-05-01658.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przedborski S., Levivier M., Jiang H., Ferreira M., Jackson-Lewis V., Donaldson D., Togasaki D. M. Dose-dependent lesions of the dopaminergic nigrostriatal pathway induced by intrastriatal injection of 6-hydroxydopamine. Neuroscience. 1995 Aug;67(3):631–647. doi: 10.1016/0306-4522(95)00066-r. [DOI] [PubMed] [Google Scholar]
- Przedborski S., Levivier M., Raftopoulos C., Naini A. B., Hildebrand J. Peripheral and central pharmacokinetics of apomorphine and its effect on dopamine metabolism in humans. Mov Disord. 1995 Jan;10(1):28–36. doi: 10.1002/mds.870100107. [DOI] [PubMed] [Google Scholar]
- Ramsay R. R., Singer T. P. Energy-dependent uptake of N-methyl-4-phenylpyridinium, the neurotoxic metabolite of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, by mitochondria. J Biol Chem. 1986 Jun 15;261(17):7585–7587. [PubMed] [Google Scholar]
- Reches A., Wagner H. R., Jiang D., Jackson V., Fahn S. The effect of chronic L-dopa administration on supersensitive pre- and postsynaptic dopaminergic receptors in rat brain. Life Sci. 1982 Jul 5;31(1):37–44. doi: 10.1016/0024-3205(82)90398-8. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Schulz R., Hodson H. F., Moncada S. Characterization of three inhibitors of endothelial nitric oxide synthase in vitro and in vivo. Br J Pharmacol. 1990 Nov;101(3):746–752. doi: 10.1111/j.1476-5381.1990.tb14151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricaurte G. A., McCann U. D. Neurotoxic amphetamine analogues: effects in monkeys and implications for humans. Ann N Y Acad Sci. 1992 May 11;648:371–382. doi: 10.1111/j.1749-6632.1992.tb24586.x. [DOI] [PubMed] [Google Scholar]
- Schulz J. B., Matthews R. T., Muqit M. M., Browne S. E., Beal M. F. Inhibition of neuronal nitric oxide synthase by 7-nitroindazole protects against MPTP-induced neurotoxicity in mice. J Neurochem. 1995 Feb;64(2):936–939. doi: 10.1046/j.1471-4159.1995.64020936.x. [DOI] [PubMed] [Google Scholar]
- Smith T. S., Swerdlow R. H., Parker W. D., Jr, Bennett J. P., Jr Reduction of MPP(+)-induced hydroxyl radical formation and nigrostriatal MPTP toxicity by inhibiting nitric oxide synthase. Neuroreport. 1994 Dec 20;5(18):2598–2600. doi: 10.1097/00001756-199412000-00048. [DOI] [PubMed] [Google Scholar]
- Viswanathan M., de Oliveira A. M., Wu R. M., Chiueh C. C., Saavedra J. M. [125I]CGP 42112 reveals a non-angiotensin II binding site in 1-methyl-4-phenylpyridine (MPP+)-induced brain injury. Cell Mol Neurobiol. 1994 Feb;14(1):99–104. doi: 10.1007/BF02088592. [DOI] [PubMed] [Google Scholar]
- Vyas I., Heikkila R. E., Nicklas W. J. Studies on the neurotoxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine: inhibition of NAD-linked substrate oxidation by its metabolite, 1-methyl-4-phenylpyridinium. J Neurochem. 1986 May;46(5):1501–1507. doi: 10.1111/j.1471-4159.1986.tb01768.x. [DOI] [PubMed] [Google Scholar]
- Walker M. J., Jenner P., Marsden C. D. A redox reaction between MPP+ and MPDP+ to produce superoxide radicals does not impair mitochondrial function. Biochem Pharmacol. 1991 Jul 25;42(4):913–919. doi: 10.1016/0006-2952(91)90053-8. [DOI] [PubMed] [Google Scholar]
- Wolff D. J., Gribin B. J. The inhibition of the constitutive and inducible nitric oxide synthase isoforms by indazole agents. Arch Biochem Biophys. 1994 Jun;311(2):300–306. doi: 10.1006/abbi.1994.1241. [DOI] [PubMed] [Google Scholar]
- Wolff D. J., Lubeskie A., Umansky S. The inhibition of the constitutive bovine endothelial nitric oxide synthase by imidazole and indazole agents. Arch Biochem Biophys. 1994 Nov 1;314(2):360–366. doi: 10.1006/abbi.1994.1454. [DOI] [PubMed] [Google Scholar]
- Zhang J., Dawson V. L., Dawson T. M., Snyder S. H. Nitric oxide activation of poly(ADP-ribose) synthetase in neurotoxicity. Science. 1994 Feb 4;263(5147):687–689. doi: 10.1126/science.8080500. [DOI] [PubMed] [Google Scholar]