Abstract
Plants are a major factor influencing methane emissions from wetlands, along with environmental parameters such as water table, temperature, pH, nutrients and soil carbon substrate. We conducted a field experiment to study how different plant species influence methane emissions from a wetland in Switzerland. The top 0.5 m of soil at this site had been removed five years earlier, leaving a substrate with very low methanogenic activity. We found a sixfold difference among plant species in their effect on methane emission rates: Molinia caerulea and Lysimachia vulgaris caused low emission rates, whereas Senecio paludosus, Carex flava, Juncus effusus and Typha latifolia caused relatively high rates. Centaurea jacea, Iris sibirica, and Carex davalliana caused intermediate rates. However, we found no effect of either plant biomass or plant functional groups – based on life form or productivity of the habitat – upon methane emission. Emissions were much lower than those usually reported in temperate wetlands, which we attribute to reduced concentrations of labile carbon following topsoil removal. Thus, unlike most wetland sites, methane production in this site was probably fuelled chiefly by root exudation from living plants and from root decay. We conclude that in most wetlands, where concentrations of labile carbon are much higher, these sources account for only a small proportion of the methane emitted. Our study confirms that plant species composition does influence methane emission from wetlands, and should be considered when developing measures to mitigate the greenhouse gas emissions.
Introduction
Wetlands are the largest natural source of the important greenhouse gas methane (CH4), contributing about one-third (80–110Tg yr−1) to global emissions [1]–[3]. This methane is produced under anoxic conditions by methanogenic microbes (Archaea) [4]. However, the amounts actually emitted from a wetland soil can be significantly influenced by vascular plants [5]–[8], through their effects upon the production, transport and consumption of methane in soils [4], [9], [10]. These processes vary greatly among plant species, and their net impact upon methane emissions can range from negative to positive [5], [10]–[12]. Much of our understanding about these processes comes from mesocosm experiments or studies conducted in environments lacking substrate uniformity. To better understand why plant species influence methane emissions differently, comparative studies are needed in wetland ecosystems under homogeneous field conditions.
In an earlier study, we showed that graminoids tended to transport more methane from rhizosphere to atmosphere than forbs [13], [14], and other workers have also found plant growth form or functional type to be a factor influencing methane emissions from wetlands [15]–[17]. However, most of this information has come from mesocosm experiments, and field studies and needs to confirm whether the observed differences are ecologically important. Furthermore, it would be useful to have more quantitative information on this topic as a basis for modelling studies and for designing mitigation strategies [18], [19].
Methane emissions from wetlands may also be influenced, either directly or indirectly, by a range of environmental factors such as water table, temperature, pH, nutrients and soil carbon [20]–[23]. For example, by affecting rates of root exudation and rhizosphere oxidation, factors influencing plant productivity could have an indirect influence upon methane emissions. Indeed, some studies have found a positive relationship of emissions with plant productivity [24], [25], while others have reported either no relationship or a negative one [26], [27]. In our mesocosm experiments, we found that plant species from less productive habitats caused higher rates of methane emission than species from more fertile habitats [10], [28]. This was apparently due to higher rates of organic acid exudation by species from less productive habitats, which increased the carbon available to methanogenic bacteria [10], [14].
Our aim in this study was to understand how plant species and/or functional plant groups (based on growth form and productivity indication values) influence methane emissions under field conditions. For this purpose, we needed a site that was as uniform as possible, to avoid possible species effects being confounded by effects due to heterogeneity in soil and hydrological conditions [29]. We therefore selected a wetland site in northern Switzerland where the top 0.5 m of soil had been removed five years previously as part of a restoration scheme, and the area sown with mixtures of native wetland plants. The species sown included both forbs and graminoids, and plants characteristic of habitats of high and low productivity. We assumed that the restoration treatment had reduced soil heterogeneity, so that emissions would be influenced more by the plant studied than by local site differences. The hypotheses were:
Plant species differ in their effect on methane emission from wetlands.
Graminoids cause higher methane emissions than forbs.
Species from low productive habitats cause higher emissions than those from high productive habitats.
Methane emissions are negatively related to plant biomass.
Materials and Methods
The experiment was conducted in a wetland (nature reserve Hütwilersee) in Eastern Switzerland (47°36′42.31” N and 8°50′15.35”E) during May 2010. The permission for the conduct of research at this site was granted by Stiftung Seebachtal, CH-Frauenfeld, the foundation owning the land and being set in-charge for the entire restoration project “Seebachtal” by the authorities of canton Thurgau. The vegetation of the study site is composed of both annual and perennial forbs and graminoids. For much of the year, the water table at the study site is at or close to the soil surface, though it may drop to 60 cm in dry periods during the summer. The site is mown once a year in autumn, and the cut material is removed. This management is similar to that practised over the centuries when the site was used as a traditional wet meadow. Preliminary tests conducted in the laboratory showed very low methanogenic activity in soil samples from the site, which increased substantially when glucose was added as a carbon source. This suggests that the unaltered substrate had very low concentrations of labile carbon, presumably as an effect of removing the top 0.5 m of soil five years previously.
The plant species studied were five forbs (Centaurea jacea L., Iris sibirica L., Lysimachia vulgaris L., Senecio paludosus L., Typha latifolia L.) and four graminoids (Carex davalliana SM., Carex flava L., Molinia caerulia (L.) Moench, Juncus effuses L.) (Table 1). Because the area was densely vegetated, there was no opportunity to make reference measurements of methane emission from bare soil. Also, no comparable site without topsoil removal was available for sampling.
Table 1. Plant species studied in the experiment ranked according to their habitat preference based on Ellenberg & Move fertility indication values.
| Species | Species code | Plant type | Ellenberg N-value* | Move N-value** |
| Molinia caerulea | MC | Graminoid (grass) | 1 | 3.15±1.11 |
| Carex davalliana | CD | Graminoid (Sedge) | 2 | - |
| Iris sibirica | IS | Forb (Monocot) | 2 | - |
| Carex flava | CF | Graminoid (Sedge) | 2 | - |
| Centaurea jacea | CJ | Forb (Dicot) | −99 | 4.76±1.01 |
| Juncus effuses | JE | Graminoid (Rush) | 4 | 4.79±1.13 |
| Lysimachia vulgaris | LV | Forb (Dicot) | −99 | 4.88±1.17 |
| Typha latifolia | TL | Forb (Monocot) | 8 | 5.95±0.90 |
| Senecio paludosus | SP | Forb (Dicot) | 6 | 6.09±0.78 |
The methane measurements were made during the last week of May 2010, using a Photo Acoustic Field Gas-Monitor type 1412 (Innova AirTech Instruments) fitted with a moisture filter [13]. The water table was recorded from the measurement tubes already installed for another experiment running in the same field. It had rained almost daily for the preceding three weeks, and the water table when the measurements were made was close to the soil surface level throughout the site. For each of the nine species, we selected six replicate spots where the plant was growing well. All spots were spread randomly within an area of 0.6 ha. At each of these spots, one or a few individuals (depending upon species) were carefully selected and covered with a transparent Plexiglas chamber (diameter 19.28 cm, height 60.17 cm), which was placed over the aboveground part of the plant(s) and was pushed about a centimetre into the moist soil for making it air tight. The change in methane concentration inside the chamber during a period of three hours was recorded, and methane emission was expressed per square meter (1 m−2) of soil surface (calculated from the diameter of the chamber).
Measurements were always made between 10:00 and 14:00 hours. Weather conditions varied from rainy to partly cloudy, and the ambient air temperature ranged between 11 and 20°C. Methane density values corresponding to air temperature were used for calculating absolute methane emissions (measured in μg m−2 hr−1). We did not extrapolate these values over longer periods because methane emissions from wetland soils are known to exhibit strong diurnal and seasonal variation [7], [30]–[32]. After each measurement, the total aboveground plant biomass from the measured spot was harvested, and a soil sample was collected from a depth of 0–30 cm. The harvested material was dried at 70°c for 48 hours and weighed to determine aboveground biomass. Soil moisture was determined by drying a portion of the sampled soil at 105°C for 24 hours. Soil pH was determined in distilled water. Nitrate and ammonium concentrations extracted with 1M KCL were determined colorimetrically using a (FIASTAR 5000) flow injection analyser [33]. During the measurement period, soil was almost always saturated with water table between 6 and 11 cm below soil surface and soil moisture (0–30 cm depth) between 86 and 89 percent. Soil pH at all sampling points was almost uniform at close to 6. Soil extractable nitrate was below detection limits, and ammonium was between 8 to 17 mg N kg−1 dry soil.
Differences among plant species in methane emissions and environmental parameters (temperature, pH, nitrogen, soil moisture) were tested by means of ANOVA and Tukey test. To confirm with the assumption of homogeneity of variance and normality, the data were log-transformed prior to data analysis. We also calculated linear regression between methane emission rates and plant biomass for each species. The data were analysed using statistical software R, version 2.8.1 [34].
Results
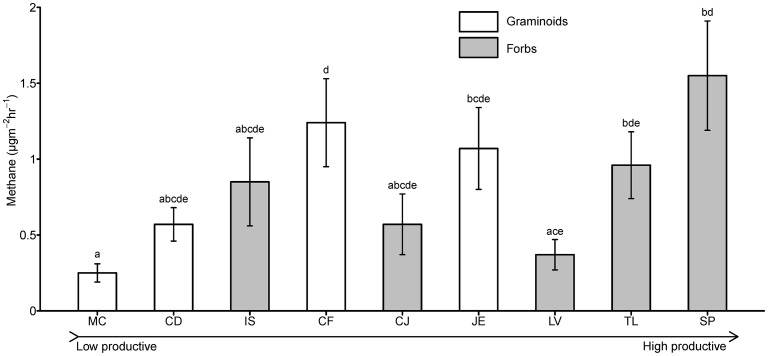
Methane emissions varied significantly among plant species (Fig. 1). Molinia caerulea was associated with the lowest emissions, while Senecio paludosus and Carex flava had the highest emissions. Emissions from Juncus effusus and Typha latifolia also tended to be higher but were statistically not different from other species. Methane emissions were sensitive to changes in air temperature, but this did not affect the comparison among different plant species since the replicates within species were spread over different days and temperatures.
Figure 1. Mean CH4 emission rates from different plant species, arranged in order of increasing fertility indication [42], [43] from left to right on x-axis (see Table 1).
MC: Molinia caerulia (L.) Moench, CD: Carex davalliana SM., IS: Iris sibirica L., CF: Carex flava L., CJ: Centaurea jacea L., JE: Juncus effuses L., LV: Lysimachia vulgaris L., TL: Typha latifolia L., SP: Senecio paludosus L. Error bars represent original data whereas statistics are based on log transformed data.
We found neither significant difference between graminoids and forbs (p = 0.95), nor between species with low and high Ellenberg N-values (below and above 4; p = 0.49). Methane emissions were also not significantly related to aboveground plant biomass; and emissions from different species were not significantly related to any of the measured site factors (Table 2).
Table 2. Results of linear regression between methane emission and environmental factors.
| Based on all data points (log-transformed) | Based on mean value per species | |||
| Factor | R squared | p-value | R squared | p-value |
| Plant Biomass | 0.005 | 0.61 | 0.011 | 0.78 |
| Soil pH | 0.004 | 0.65 | 0.049 | 0.56 |
| Soil extractable NH4– Nitrogen | 0.009 | 0.49 | 0.120 | 0.35 |
| Air Temperature | 0.088 * | 0.03 | 0.001 | 0.92 |
| Soil moisture | 0.001 | 0.86 | 0.112 | 0.37 |
| Water table | 0.012 | 0.42 | 0.075 | 0.47 |
Soil variables were measured in the top 30 cm.
P≤0.05; **P≤0.01; ***P≤0.001.
Discussion
Methane emissions from natural wetlands have been reported to range between 0 and 660 mg m−2 day−1 [1], [2], [30], [35]. At our site, they varied from 0.25 to 1.55 μg m−2 hr−1, which is lower by a magnitude of thousands than those measured at many other temperate wetlands [27], [36]. Moore and Knowles [37] have reported similarly low rates of emission from an ombrotrophic bog in Quebec (Canada), where they were perhaps caused by very slow decomposition of Sphagnum litter and suboptimal pH for methanogenesis. At our site, the low methanogenic activity was probably a consequence of removing the topsoil five years previously, thereby reducing concentrations of labile carbon in the substrate. Indeed, in a separate mesocosm study, we found that methane emissions increased dramatically when glucose was added to the substrate obtained from the same field site. In addition, the site was mown each year and the hay removed, so that little aboveground biomass entered the soil as litter. Thus, unlike most wetland sites, methane production in this site was probably fuelled chiefly by root exudation from living plants and from root decay [38]. The very low overall methane emission, which is one of the main results from our study, underlines an important aspect of wetland management and methane mitigation. It suggests that topsoil removal coupled with regular biomass removal can reduce methane emissions for an extended period – although we can only base this conclusion on comparison with other sites since control sites without topsoil removal were not available in our site. Furthermore, it must be noted that we measured methane emission on only one occasion, chosen for the hydrological homogeneity caused by several weeks of continuous rain.
Despite the low emissions, the site was well suited for comparing methane emissions associated with different plant species. In support of our first hypothesis, methane emission from soil at various points varied significantly according to the plant species growing there. Previous studies have also shown that plant species differ in their influence on methane emissions, though these were conducted either in mesocosms [10], [13], [17], [22] or under rather heterogeneous field conditions [8], [11] where microtopographic or other factors might have affected the results [5], [36]. In our field site, we found no correlation between environmental parameters and methane emissions from different plant species, presumably because of the rather uniform site conditions [20], [23].
In an earlier greenhouse experiment comparing a large set of wetland plants, we found that graminoids had a greater capacity to transport methane from soil to atmosphere (chimney effect) than forbs [13]. This effect appeared to be largely a result of differences between the root systems in the two functional groups [39], [40]. No such relationship was evident in the current study, however, presumably because too little methane was produced to detect any differences in transport capacity.
Results of this field study do not support our third and fourth hypothesis, which were based on mesocosm experiments showing higher emissions caused by species of less productive habitats and decreasing emissions with increase in plant productivity [10], [26]–[28]. Higher methane emission from mesocosms with species adapted to less productive habitats was attributed to higher carbon exudation rates in these species [10]. Because our study site was depleted of labile carbon, we had expected to find similar effects in the field, arguing that under these conditions differences in exudation rates among species would be detectable as differences in methane emissions. But we found neither a difference among plant species from low and high productive habitats nor a significant relationship between methane emissions and plant biomass (Table 2). Some field studies have shown a positive correlation between methane emissions and vegetation biomass or productivity [12], [24], [25], while mesocosm experiments have shown the opposite effect [10], [27], [28]. This difference may have arisen because the amount of methane actually emitted is the resultant of several processes, but which of these dominates may vary according to the conditions. For example, under conditions at our site, the addition of carbon through root exudation was probably the primary controlling mechanism, which in turn depended upon current photosynthetic activity of the vegetation [24], [41]. In contrast, when plants are grown under controlled conditions such as in mesocosm experiments, the rhizosphere oxidation by the plant roots might be of relatively greater significance [26], and therefore plants with larger biomass are able to exert a relatively larger influence through radial oxygen loss by occupying all the rhizosphere space available in the mesocosm. Thus, mesocosm experiments do not necessarily reflect processes operating in the field.
In conclusion, this study demonstrates that plant species vary in their capacity to influence methane emissions even under conditions where methanogenesis is limited by a shortage of labile carbon. However, the very low fluxes measured in our study suggest that only a small proportion of the methane emitted from most wetlands can be due to root exudation. This in turn suggests that plants have a much greater potential to influence emissions by transporting methane internally or by altering redox conditions in the soil. Finally, our results indicate that management practices such as removing topsoil and mowing the vegetation could be an effective way of reducing methane emissions from wetlands. Further research would be needed, however, to determine whether any benefits would exceed the ‘carbon debt’ associated with removing the top soil.
Acknowledgments
We thank Matthias Suter and Dieter Ramseier for allowing us to use the study site and for providing historic data on hydrology and management of the site; and Matthew Scarborough for helping with conduct of the experiment.
Funding Statement
This work was supported by ETH Zurich [TH-07 07-3]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Aselmann I, Crutzen PJ (1989) Global distribution of natural freshwater wetlands and rice paddies, their net primary productivity, seasonality and possible methane emissions. J Atmos Chem 8: 307–358. [Google Scholar]
- 2. Cao M, Gregson K, Marshall S (1998) Global methane emission from wetlands and its sensitivity to climate change. Atmos Environ 32: 3293–3299. [Google Scholar]
- 3.IPCC (2007) Climate Change 2007; Solomon S, Qin D, Manning M, Chen Z, Marquis M, et al., editors. Cambridge, UK and New York, USA: Cambridge University Press.
- 4. LeMer J, Roger P (2001) Production, oxidation, emission and consumption of methane by soils: A review. Eur J Soil Biol 37: 25–50. [Google Scholar]
- 5. Rothman E, Bouchard V (2007) Regulation of carbon processes by macrophyte species in a Great Lakes coastal wetland. Wetlands 27: 1134–1143. [Google Scholar]
- 6. Lai DYF (2009) Methane Dynamics in Northern Peatlands: A Review. Pedosphere 19: 409–421. [Google Scholar]
- 7. Ding WX, Cai ZC, Tsuruta H, Li XP (2003) Key factors affecting spatial variation of methane emissions from freshwater marshes. Chemosphere 51: 167–173. [DOI] [PubMed] [Google Scholar]
- 8. Stanley E, Ward A (2010) Effects of Vascular Plants on Seasonal Pore Water Carbon Dynamics in a Lotic Wetland. Wetlands 30: 889–900. [Google Scholar]
- 9. Joabsson A, Christensen TR, Wallen B (1999) Influence of vascular plant photosynthetic rate on CH4 emission from peat monoliths from southern boreal Sweden. Polar Res 18: 215–220. [Google Scholar]
- 10. Koelbener A, Ström L, Edwards PJ, Olde Venterink H (2010) Plant species from mesotrophic wetlands cause relatively high methane emissions from peat soil. Plant Soil 326: 147–158. [Google Scholar]
- 11. Ding WX, Cai ZC, Tsuruta H (2005) Plant species effects on methane emissions from freshwater marshes. Atmos Environ 39: 3199–3207. [Google Scholar]
- 12. Joabsson A, Christensen TR (2001) Methane emissions from wetlands and their relationship with vascular plants: an Arctic example. Glob chang Biol 7: 919–932. [Google Scholar]
- 13. Bhullar GS, Edwards PJ, Venterink HO (2013) Variation in the plant-mediated methane transport and its importance for methane emission from intact wetland peat mesocosms. J Plant Ecol 6: 298–304. [Google Scholar]
- 14. Bhullar GS, Iravani M, Edwards PJ, Olde Venterink H (2013) Methane transport and emissions from soil as affected by water table and vascular plants. BMC Ecol 13: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sebacher DI, Harriss RC, Bartlett KB (1985) Methane emissions to the atmosphere through aquatic plants. J Environ Qual14: 40–46. [Google Scholar]
- 16. Schimel JP (1995) Plant-transport and methane production as controls on methane flux from arctic wet meadow tundra. Biogeochemistry 28: 183–200. [Google Scholar]
- 17. Rice AL, Butenhoff CL, Shearer MJ (2010) Teama D, Rosenstiel TN, et al (2010) Emissions of anaerobically produced methane by trees. Geophys Res Lett 37: L03807. [Google Scholar]
- 18. Ward SE, Bardgett RD, McNamara NP, Ostle NJ (2009) Plant functional group identity influences short-term peatland ecosystem carbon flux: evidence from a plant removal experiment. Func Ecol 23: 454–462. [Google Scholar]
- 19. Dias ATC, Hoorens B, Van Logtestijn RSP, Vermaat JE, Aerts R (2010) Plant Species Composition Can Be Used as a Proxy to Predict Methane Emissions in Peatland Ecosystems After Land-Use Changes. Ecosystems 13: 526–538. [Google Scholar]
- 20. Christensen TR, Ekberg A, Strom L, Mastepanov M, Panikov N, et al. (2003) Factors controlling large scale variations in methane emissions from wetlands. Geophys Res Lett 30: 67 (61–64).. [Google Scholar]
- 21. Waddington JM, Roulet NT (1996) Atmosphere-wetland carbon exchanges: Scale dependency of CO2 and CH4 exchange on the developmental topography of a peatland. Global Biogeoch Cycles 10: 233–245. [Google Scholar]
- 22. Dinsmore KJ, Skiba UM, Billett MF, Rees RM (2009) Effect of water table on greenhouse gas emissions from peatland mesocosms. Plant Soil 318: 229–242. [Google Scholar]
- 23. Inubushi K, Otake S, Furukawa Y, Shibasaki N, Ali M, et al. (2005) Factors influencing methane emission from peat soils: Comparison of tropical and temperate wetlands. Nutr Cycl Agroecosys 71: 93–99. [Google Scholar]
- 24. Chanton JP, Whiting GJ, Blair NE, Lindau CW, Bollich PK (1997) Methane emission from rice: Stable isotopes, diurnal variations and CO2 exchange. Global Biogeoch Cycles 11: 15–27. [Google Scholar]
- 25. Whiting GJ, Chanton JP (1993) Primary production control of methane emissions from wetlands. Nature 364: 794–795. [Google Scholar]
- 26. Ström L, Mastepanov M, Christensen TR (2005) Species-specific effects of vascular plants on carbon turnover and methane emissions from wetlands. Biogeochemistry 75: 65–82. [Google Scholar]
- 27. Bouchard V, Frey SD, Gilbert JM, Reed SE (2007) Effects of macrophyte functional group richness on emergent freshwater wetland functions. Ecology 88: 2903–2914. [DOI] [PubMed] [Google Scholar]
- 28.Bhullar GS (2011) The role of plants in the production and transport of methane from wetland soils [PhD Dissertation]. Zurich: Swiss Federal Institute of Technology (ETH), Zurich. 95 p. [Google Scholar]
- 29. Bubier JL (1995) The relationship of vegetation to methane emission and hydrochemical gradients in northern peatlands. J Ecol 83: 403–420. [Google Scholar]
- 30. Moore T, Roulet N, Knowles R (1990) Spatial and temporal variations of methane flux from subarctic/northern boreal fens. Global Biogeoch Cycles 4: 29–46. [Google Scholar]
- 31. Long KD, Flanagan LB, Cai T (2010) Diurnal and seasonal variation in methane emissions in a northern Canadian peatland measured by eddy covariance. Glob Chang Biol 16: 2420–2435. [Google Scholar]
- 32. Schutz H, Holzapfelpschorn A, Conrad R, Rennenberg H, Seiler W (1989) A 3-year continuous record on the influence of daytime, season, and fertilizer treatment on methane emission rates from an Italian rice paddy. Journal of J Geophys Res-Atmos 94: 16405–16416. [Google Scholar]
- 33.Maynard DG, Kalra YP, Crumbaugh JA (2008) Nitrate and exchangeable ammonium nitrate. In: Carter MR, Geregorich EG, editors. Soil sampling and methods of analysis. 2 ed: Canadian society of soil scientists. 71–80.
- 34.R Development Core Team (2008) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria: http://www.r-project.org/.
- 35. Morrissey LA, Livingston GP (1992) Methane emissions from Alaska arctic tundra- an assessment of local spatial variability. J Geophys Res-Atmos 97: 16661–16670. [Google Scholar]
- 36. Bubier JL, Moore TR, Bellisario L, Comer NT, Crill PM (1995) Ecological controls on methane emissions from a northern peatland complex in the zone of discontinuous permafrost, Manitoba, Canada. Global Biogeoch Cycles 9: 455–470. [Google Scholar]
- 37. Moore TR, Knowles R (1990) Methane Emissions from Fen, Bog and Swamp Peatlands in Quebec. Biogeochemistry 11: 45–61. [Google Scholar]
- 38. Saarnio S, Wittenmayer L, Merbach W (2004) Rhizospheric exudation of Eriophorum vaginatum L. – Potential link to methanogenesis. Plant Soil 267: 343–355. [Google Scholar]
- 39. Hirota M, Tang YH, Hu QW, Hirata S, Kato T, et al. (2004) Methane emissions from different vegetation zones in a Qinghai-Tibetan Plateau wetland. Soil Biol & Biochem 36: 737–748. [Google Scholar]
- 40. Torn MS, Chapin FS (1993) Environmental and biotic controls over methane flux from arctic tundra. Chemosphere 26: 357–368. [Google Scholar]
- 41. Chanton JP, Bauer JE, Glaser PA, Siegel DI, Kelley CA, et al. (1995) Radiocarbon Evidence for the Substrates Supporting Methane Formation within Northern Minnesota Peatlands. Geochim Cosmochim Acta 59: 3663–3668. [Google Scholar]
- 42. Ellenberg H, Weber HE, Düll R, Wirth V, Werner W, et al. (1991) Zeigerwerte von Pflanzen in Mitteleuropa. Scripta Geobotanica 18: 1–248. [Google Scholar]
- 43.Bakkenes MD, De Zwart D, Alkemade JRM (2002) MOVE nationaal Model voor de Vegetatie versie 3.2 Achtergronden en analyse van modelvarianten. IVM, Bilthoven – Nederland: 1–124.