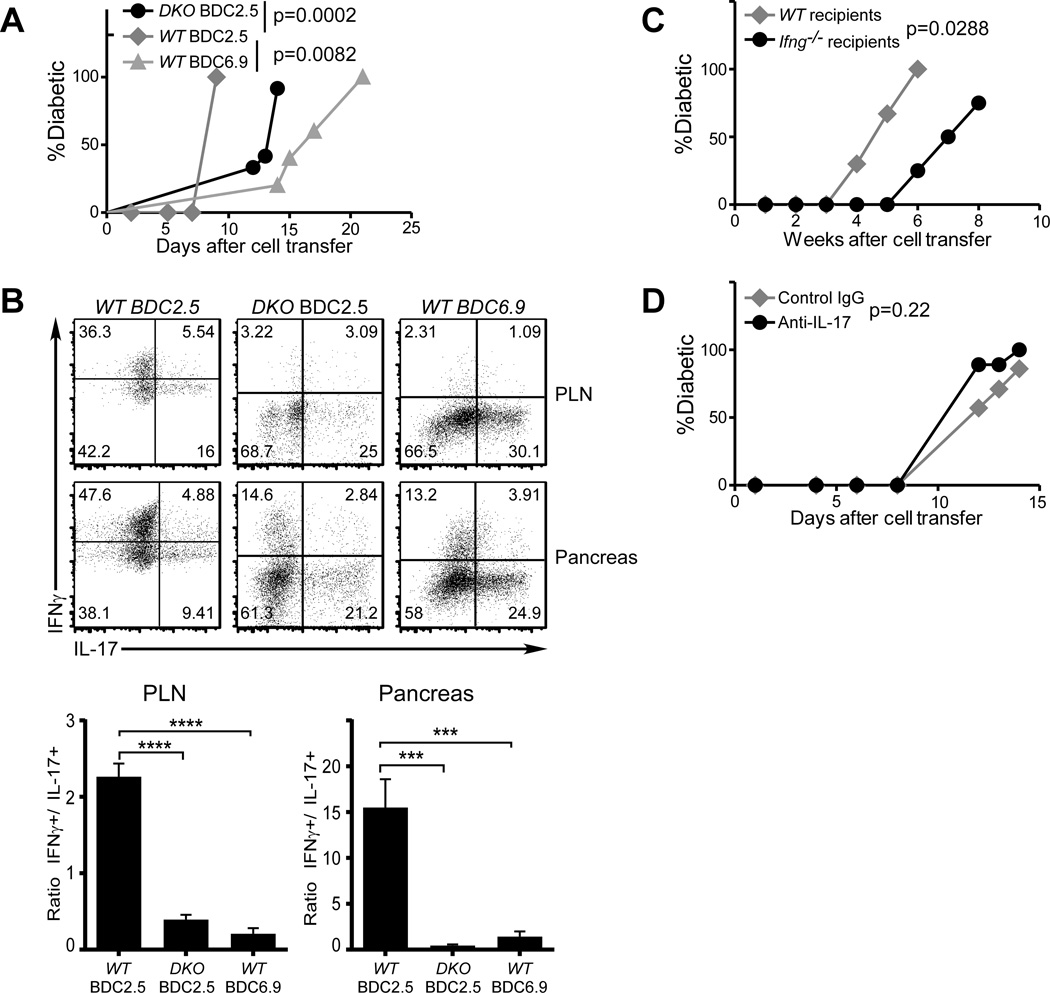
FIGURE 4. Neither Reprogramming nor IFNγ is required for the pathogenicity of islet antigen-specific Th17 cells.
(A and B) Purified in vitro differentiated WT BDC2.5 (gray diamonds), WT BDC6.9 (gray triangles), or Ifngr−/−Stat4−/− BDC2.5 (DKO, black circles) Th17 cells were transferred into NOD.Scid recipients (0.5×106/recipient, n=5/group). Diabetes incidence was monitored (A). Upon disease onset, the donor cells were analyzed for cytokine expression (B, upper panels, representative from each group). The ratios between IFNγ+ and IL-17+ donor cells were calculated (B lower panels). (C) Purified in vitro differentiated Ifng−/− BDC2.5 Th17 cells were transferred into WT NOD (gray diamonds) or NOD.Ifng−/− black circles) recipients (0.5×106 per recipient, n=3 for WT, n=4 for Ifng−/−). Diabetes incidence was monitored after cell transfer. (D) Purified in vitro differentiated BDC2.5 Th17 cells were transferred into NOD.Scid recipients (0.5×106 per recipient). The recipients were then treated with anti-IL-17 neutralizing antibody (black circles) or control mouse IgG (gray diamonds) (300µg/dose and 2 doses/week, 5 recipients per group). Diabetes incidence was monitored after cell transfer.