Abstract
Objectives
Influenced by pathogen exposure and obesity, inflammation provides a critical biological pathway linking changing environments to the development of cardiometabolic disease. This study tests the relative contribution of obesogenic and pathogenic factors to moderate and acute CRP elevations in Chinese children, adolescents and adults.
Methods
Data come from 8795 participants in the China Health and Nutrition Study. Age-stratified multinomial logistic models were used to test the association between illness history, pathogenic exposures, adiposity, health behaviors and moderate (1-10 mg/L in children and 3-10 mg/L in adults) and acute (>10mg/L) CRP elevations, controlling for age, sex and clustering by household. Backward model selection was used to assess which pathogenic and obesogenic predictors remained independently associated with moderate and acute CRP levels when accounting for simultaneous exposures.
Results
Overweight was the only significant independent risk factor for moderate inflammation in children (RRR 2.10, 95%CI 1.13-3.89). History of infectious (RRR 1.28, 95%CI 1.08-1.52) and non-communicable (RRR 1.37, 95%CI 1.12-1.69) disease, overweight (RRR 1.66, 95%CI 1.45-1.89) and high waist circumference (RRR 1.63, 95%CI 1.42-1.87) were independently associated with a greater likelihood of moderate inflammation in adults while history of infectious disease (RRR 1.87, 95%CI 1.35-2.56) and overweight (RRR 1.40, 95%CI 1.04-1.88) were independently associated with acute inflammation. Environmental pathogenicity was associated with a reduced likelihood of moderate inflammation, but a greater likelihood of acute inflammation in adults.
Conclusions
These results highlight the importance of both obesogenic and pathogenic factors in shaping inflammation risk in societies undergoing nutritional and epidemiological transitions.
Keywords: C-reactive protein, Inflammation, Obesity, Infectious Disease, China, Life course
Exposure to changing social and physical environments has been implicated as an important factor in the increasing global prevalence of obesity and chronic disease (Popkin and Gordon-Larsen 2004; Swinburn et al. 2011; Yusuf et al. 2001). The mechanisms driving these associations, however, are not fully understood. Inflammation may provide a critical biological pathway linking changing environments to the development of cardiometabolic disease. C-reactive protein (CRP), a systemic inflammatory biomarker, is related to the numerous social, behavioral and biological factors -such as socioeconomic status (Gimeno et al. 2008; Nazmi et al. 2010b), exposure to environmental pathogens (Kushner et al. 2006; McDade et al. 2005; McDade et al. 2008), and abdominal adiposity (McDade et al. 2008; Park et al. 2005) - that accompany urbanization and modernization of diets and lifestyles. Previous research suggests that both chronic pathogen exposure and increasing adiposity activate pro-inflammatory pathways (McDade et al. 2008; Vahdat et al. 2012) and contribute to cardiometabolic disease risk (Dowd et al. 2009; Zhu et al. 2000). Infection with common pathogens has been linked to higher CRP levels in American children (Dowd et al. 2010) and adults (Nazmi et al. 2010a; Zhu et al. 2000), with greater risk of inflammation seen with increasing pathogen burden. Similarly, higher body weight and body mass index have been associated with chronic inflammation in children (Choi et al. 2013; Cook et al. 2000; Dowd et al. 2010) and adults (Choi et al. 2013; Kushner et al. 2006), with the risk of inflammation increasing with higher weight status (Nguyen et al. 2009; Visser et al. 1999).
The majority of research on inflammation comes from developed countries where levels of obesity are high and levels of infection are comparatively low. The associations between chronic infection, overweight, and inflammation may be especially problematic for individuals in developing countries, who confront a dual burden of prevalent chronic disease and increasing rates of overweight and obesity alongside infectious disease and early life malnutrition. While concerns have been raised that the nutrition transition places individuals at increased risk for the development of inflammation and subsequent cardiometabolic disease (Gurven et al. 2009; McDade 2012), relatively little is known about the dynamics of inflammation in populations facing simultaneous exposure to pathogenic and obesogenic environments. Previous research in the Philippines documented that pathogen exposure and measures of adiposity are significant independent predictors of inflammation (McDade et al. 2008; 2009a). However, the impact of these simultaneous exposures remains relatively unstudied in comparable contexts and across a wide range of ages and environments.
We explore the pathogenic and obesogenic factors associated with inflammation in children, adolescents, and adults participating in the China Health and Nutrition Survey (CHNS; Popkin et al. 2010). China represents an important context for addressing the environments placing individuals at risk for inflammation across the life course. Its unprecedented rates of urbanization (Zhang and Song 2003) and economic growth (Ma 2002) mean that dramatic social and environmental change have occurred within an single generation. Accompanying these socioeconomic changes have been marked changes in diet (Du et al. 2004; Wang et al. 2008), physical activity (Monda et al. 2008; Ng et al. 2009) and morbidity (Wang et al. 2007; Yoon et al. 2006). China's morbidity and mortality profile has only recently shifted from one dominated by infectious disease to one dominated by obesity and cardiometabolic disease (He et al. 2005; Popkin et al. 2006). Although environmental and economic change has been ubiquitous, the patterns and rates of change have not been universal (Jones-Smith and Popkin 2010; Li et al. 2012); rather, substantial urban/rural differences exist in environmental quality and causes of morbidity (Gu et al. 2005; Zhu et al. 2011). Differences in CRP have been documented by region and urban/rural residence in Chinese adults (Ye et al. 2007; Zhao et al. 2010). However, no previous studies in China have examined individual- and household-level environmental and behavioral correlates of inflammation.
Identification of pro-inflammatory factors is critical in contexts like China, where simultaneous pathogenic and obesogenic environmental exposures may create a dual burden of inflammation at even young ages. Using household-level measures of environmental pathogenicity and individual-level anthropometric, health and behavior data from 8795 CHNS participants, we test factors within the two pathways --pathogenic and obesogenic-- previously linked to inflammation. Because we are interested in the range of environments and behaviors that may place individuals at risk, we consider both proximate (e.g. illness symptoms and overweight) and more distal (e.g. household sanitation and diet) factors within each of these pathways. We hypothesize that exposure to environmental pathogens, measured through household sanitation and individual illness history, will be associated with acute elevations in CRP. Further, we would expect to see this pattern more strongly in children and adolescents with higher exposures in rural areas, since children tend to be more vulnerable to environmental pathogens (DeBoer et al. 2012). Second, we hypothesize that moderate elevations in CRP will be associated with obesogenic exposures, such as high BMI and waist circumference, that are increasingly common among adults living in urban areas. Since CRP is produced by the liver in response to cytokine production by visceral adipose tissue (Park et al. 2005; Schaffler et al. 2006), other behaviors contributing to the accumulation of visceral fat, such as high fat diets and low physical activity, would also be expected to be associated with elevated CRP. Finally, we hypothesize that, given the rapidity of change in China, a combination of factors within both of these pathways will be present simultaneously and test how this simultaneous exposure relates to the relative risk of moderate and acute elevations in CRP.
Sample and methods
The China Health and Nutrition Survey (CHNS)
Survey procedures have been previously described in detail (Popkin et al. 2010). Briefly, the CHNS has collected community-, household-, and individual-level environmental, health and dietary data from over 14,000 individuals from over 4400 households across nine diverse provinces (Guangxi, Guizhou, Heilongjiang, Henan, Hubei, Hunan, Jiangsu, Liaoning, and Shandong) in eight waves from 1989-2009. The original survey in 1989 used a multistage, random cluster design to select a stratified probability sample that was diverse in urban/rural location and socioeconomic status. Using this sampling strategy, two cities, one large and one small city --usually the provincial capital and a lower income city-- and four counties stratified by income --one high, one low, and two middle income counties-- were selected per province. Within cities, two urban and two suburban communities were randomly selected; within counties, one community in the capital city and three rural villages were randomly chosen. Twenty households per community were then randomly selected for participation. Data were collected in the households during three days of visits, which included sociodemographic surveys, anthropometry, 24-hour dietary recall and weighed food inventories, and household environmental measures. Blood samples were collected in participants over the age of 7 at neighborhood clinics or, in cases where participants were unable to attend the clinic visit, at home.
The current analysis focuses on a cross-sectional sample from the 2009 wave, the first to collect biomarkers. This analytic sample includes 795 children and adolescents aged 7-18 (n=438 males) and 8615 adults aged 18-99 (n=4031 males) living in 4246 households. Individuals were included in the study if they had collected biomarkers at the 2009 exam, representing 93% of the total sample. This study protocol and analysis was approved by the Institutional Review Board at the University of North Carolina at Chapel Hill, the China-Japan Friendship Hospital, Ministry of Health and China, and Institute of Nutrition and Food Safety, China Centers for Disease Control. Subjects gave informed consent for participation.
Measures
High-Sensitivity C-Reactive Protein (hs-CRP)
Fasting blood samples were collected from all participants aged 7 and older by venipuncture. All samples were analyzed in a nationally accredited medical laboratory in Beijing (ISO 15189:2007). Serum CRP levels were measured using a high sensitivity immunoturbidmetric method (Hitachi 7600 automated analyzer) with Denka (Seiken, Japan) reagents. This assay has a range of 0.1-320 mg/L and a coefficient of variation <7.0% across assays. The distribution of CRP was highly skewed even after logarithmic transformation; consequently, CRP was categorized into a 3-level variable representing normal, moderate, and acute inflammation. Moderate elevations in CRP were defined as values between 1-10 mg/L for children (Skinner et al. 2010) and 3-10mg/L for adults (Pearson et al. 2004). Acute inflammation was defined as CRP values >10mg/L for both children and adults (Pearson et al. 2004). Sensitivity analysis was conducted with alternate cut-points of 1-5 mg/L and >5mg/L in children and adolescents (McDade et al. 2005) and 2-10 mg/L and >10mg/L in adults (Zhao et al. 2010) due to concerns with the appropriateness of the established cut-points in non-Western contexts (Wander et al. 2012).
Environmental pathogenicity
Urban/rural residence was defined by government classifications of community boundaries within municipalities or counties. Total household income, measured in yuan, was divided into tertiles, and, following previous analyses with this sample (Yan et al. 2012), the low-income group was compared to a combined medium/high income group for ease of interpretation. Information on water sources, housing conditions and amenities, economic activities, and household assets were collected during the household visit. For the present analysis, we assessed whether any household members had raised livestock in the past year, whether the home had access to running water, the presence of a toilet in the home, and whether the interviewer saw excrement around the home. Although direct measures of air quality are not available for the CHNS, we created two proxy measures of local air pollution: type of cooking fuel used and exposure to car and motorcycle exhaust. Cooking fuel exposure was defined dichotomously as exposure to particulate-generating fuels (coal, charcoal and wood) vs. non-particulate-generating fuels (electricity, natural gas and kerosene). Exposure to car and motorcycle exhaust was created at the community-level by dividing the total number of cars and motorcycles by the number of households. Each household was dichotomized into low or high exposure based on whether their community was above or below the median community density.
Self-reported illness symptoms experienced during the month preceding the home visit were used as a measure of environmental pathogenicity and disease burden. Participants were asked whether they had had any illnesses or injuries over the past month and about the occurrence of 11 symptoms/conditions (fever/sore throat/cough, diarrhea, stomachache, asthma, headache/dizziness, joint/muscle pain, rash, eye/ear disease, heart disease/chest pain, other infectious disease, and other noncommunicable disease). We used these symptoms to create an infectious illness summary variable, assigning a positive value when the participant had experienced fever/cough/sore throat, diarrhea or other infectious illness, and a noncommunicable illness summary variable, assigning a positive value when the participant had experienced heart disease/chest pain or other noncommunicable illness symptoms. We also separately examined several symptoms previously associated with elevated CRP in children and adults, including fever/cough/sore throat, diarrhea, heart conditions, and asthma (Filteau et al. 1995; Kushner et al. 2006; McDade et al. 2009b; Shima 2007). In addition to these one-month recall measures, participants were asked if they were sick or had experienced a limited set of symptoms in the 24-hours preceding blood collection. We examined these 24-hour variables as both an alternative measure and also as a potential confounder in the final combined models. While having experienced infectious symptoms in the past 24 hours was individually and independently associated with a significantly higher risk of acutely elevated CRP in children and adults, the inclusion of these 24-hr recall variables (any illness, any infectious illness, or any non-communicable illness) instead of or in addition to the one-month measures did not change the presented results. As a result, we retained the one-month variables as more comprehensive summary measure of overall disease burden.
Individual measures
Weight, height, and waist circumference were collected by two trained study personnel following standard protocols. Weight was measured to the nearest 0.1 kg on a calibrated beam scale. Height was measured to the nearest 0.1 cm using a portable Seca stadiometer. Waist circumference was measured midway between the lowest rib and the iliac crest using a non-elastic tape. BMI was calculated as kg/m2. Overweight and obesity were defined using the WHO cut-points of 25 kg/m2 and 30 kg/m2 for adults and the International Obesity Task Force (IOTF) age-specific equivalent values for children. We chose these WHO definitions of overweight/obesity in adults rather than the Chinese-specific cut-points for comparability across children and adults. High waist circumference in adults was defined using Asia-specific cut-points of >80cm for women and >90cm for men (Alberti et al. 2006). High waist-circumference in children and adolescents was defined as a waist circumference above the sample-specific 85th percentile for age.
Physical activity was reported using a detailed 7-day physical activity recall instrument. Leisure activities were assigned Metabolic Equivalent (MET) values using the Compendium of Physical Activity (Ainsworth et al. 2000), which has been previously used in China (Monda et al. 2008; Ng and Popkin 2012). Active was defined as participating in ≥27 METs per week of leisure time physical activity, equivalent to ≥1 hour per week of moderate-intensity exercise such as brisk walking. Dietary fat intake was derived from individual-level 24-hour recalls taken on three consecutive days and adjusted for expected individual consumption from the weighed household inventory if there was a large difference in consumption between the household and individual levels (Du et al. 2004; Popkin et al 2002; Zhai et al. 1996). Macronutrient values were estimated from the 2002/2004 Chinese Food Composition Table (FCT) and diets were considered high fat if 30% or more of the total daily energy intake was derived from fat (Du et al. 2004). Smoking was included as a dichotomous variable for adults using participant responses to the question of whether they were currently smoking at the 2009 survey. This variable was not included in the child models due to the low prevalence of smoking in younger children (<2%).
Statistical analysis
Descriptive statistics were used to assess differences in our main individual-level exposures by age, gender and urban/rural status and to test for household-level differences in environmental exposures by urban/rural residence. Chi square tests were also used to test for differences in the prevalence of moderate and acute inflammation by residence and income. We conducted two sets of analyses to examine the pathogenic and obesogenic predictors of inflammation. First, a series of age-stratified multinomial logistic regression models comparing moderate and acute CRP elevation to normal CRP were run separately for the individual pathogenic and obesogenic predictors of interest controlling for age strata (7-12.99 and 13-17.99 for children and adolescents and 18-39.99, 40-59.99, and >60 for adults) and sex. Estimates and standard errors were adjusted to control for clustering by household. In these multinomial models, the relative risk ratio is interpreted as the likelihood that an individual with the exposure, such as not having a toilet in the home or eating a high fat diet, will have moderately or acutely elevated CRP versus normal CRP levels, holding other variables constant. These models were run separately due to correlations between exposure variables and our interest in describing the range of potential pathogenic and obesogenic exposures. Next, backward model selection was used to determine which factors remained independently associated with inflammation when accounting for simultaneous exposure to pathogenic and obesogenic factors. All variables, including urban/rural residence, income, the illness summary measures, household environment measures, anthropometry, diet and physical activity, were entered into the model with a removal probability of p<0.10. The final selected models are adjusted for age strata, sex, and clustering by household. All statistical analysis was performed with STATA software (version 12; STATA Corporation, College Station, TX).
Results
Sample Characteristics
Individual-level explanatory and exposure variables are summarized in Table 1 by age, sex, and urban/rural residence. The prevalence of overweight/obesity and high waist circumference was higher in adults than children and adolescents and showed few significant differences by urban/rural residence. Obesity prevalence differed between urban and rural men with significantly higher prevalence seen in urban men (5.4% vs. 3.2%, respectively), while the prevalence of high waist circumference was higher in urban than rural girls (13.64% vs. 6.81%, respectively). Diet and physical activity showed more consistent urban/rural differences in both children and adults. With the exception of leisure time physical activity, which did not significantly differ between rural and urban girls, the prevalence of high fat diets and leisure activity were higher in urban areas for all age and sex strata. Men reported much higher rates of smoking than women, 52-56% compared to 3-4%, respectively, with the highest prevalence seen in rural men. Finally, illness history also differed across age, sex, and residence. Urban girls and women reported significantly higher rates of illness in the past four weeks compared to their rural counterparts, with more urban girls reporting infectious illness symptoms and more urban women reporting non-communicable disease symptoms.
Table 1. Sample characteristics.
| Children and Adolescents | Adults | |||||||
|---|---|---|---|---|---|---|---|---|
| Rural | Urban | Rural | Urban | |||||
| Boys | Girls | Boys | Girls | Men | Women | Men | Women | |
| Mean (STD)/% (N) | Mean (STD)/% (N) | Mean (STD)/% (N) | Mean (STD)/% (N) | Mean (STD)/% (N) | Mean (STD)/% (N) | Mean (STD)/% (N) | Mean (STD)/% (N) | |
| N | 337 | 245 | 101 | 112 | 2715 | 3072 | 1316 | 1512 |
| Age (yrs) | 12.07 (2.80) | 12.05 (2.76) | 12.21 (3.18) | 12.43 (2.85) | 50.37 (14.91) | 50.23 (15.01) | 51.58 (15.59)* | 51.92 (15.20) |
| Body Mass Index (kg/m²) | 17.57 (3.23) | 17.61 (3.41) | 18.36 (3.82)* | 17.87 (2.97) | 23.21 (3.31) | 23.38 (3.54) | 23.60 (3.57)** | 23.43 (3.53) |
| % Overweighta | 11.69 (38) | 9.58 (23) | 14.58 (14) | 10.81 (12) | 25.17 (671) | 25.13 (748) | 26.68 (341) | 25.90 (381) |
| % Obeseb | -- | -- | -- | -- | 3.23 (86) | 4.67 (139) | 5.40 (69)** | 4.08 (60) |
| Waist circ, cm | 63.92 (10.22) | 61.64 (9.26) | 63.82 (9.81) | 63.06 (7.64) | 83.99 (10.26) | 81.42 (10.31) | 85.27 (10.17)** | 80.99 (10.26) |
| % High WCc | 15.29 (50) | 6.81 (16) | 12.77 (12) | 13.64 (15)* | 30.17 (803) | 55.09 (1,634) | 31.72 (406) | 53.88 (791) |
| % High-fat dietd | 41.77 (137) | 42.39 (103) | 61.05 (58)** | 63.06 (70)** | 40.53 (1,089) | 47.00 (1,420) | 62.11 (808)** | 65.44 (977)** |
| % Activee | 28.30 (88) | 15.52 (36) | 42.86 (39)** | 23.85 (26) | 26.93 (727) | 20.20 (616) | 49.81 (652)** | 39.83 (599)** |
| % Current smoker | -- | -- | -- | -- | 55.54 (1,499) | 3.68 (113) | 52.07 (678)* | 3.38 (51) |
| % Sick in past 4 weeksf | 9.79 (33) | 8.20 (20) | 12.87 (13) | 18.02 (20)** | 17.02 (462) | 19.10 (587) | 18.92 (249) | 21.63 (327)* |
| % Infectious Symptomsg | 8.31 (28) | 7.38 (18) | 11.88 (12) | 16.22 (18)* | 9.40 (255) | 9.73 (299) | 11.32 (149) | 11.11 (168) |
| % Non-Communicable Symptomsh | 0.59 (2) | 0.41 (1) | 0 (0) | 0 (0) | 5.60 (152) | 6.05 (186) | 6.76 (89)* | 9.13 (138)** |
p<0.05;
p<0.01; for difference between urban and rural residents within sex and age strata
Overweight defined as a BMI between 25-30 kg/m2 for adults or above the age-adjusted IOTF equivalent of 25 kg/m2 for children.
Obesity defined as a BMI >30 kg/m2
Asia-specific cut-point of 90cm for men and 80cm for women (Aliberti et al. 2003).
>30% of energy from fat (Du et al., 2004)
The equivalent of >1 hour of moderate-vigorous leisure time activity per week.
Presence of any self-reported illness symptom in the 4 weeks prior to blood collection.
Includes fever/cough/sore throat, diarrhea and “other infectious disease” symptoms experienced in the 4 weeks prior to blood collection
Includes heart problems and “other non-communicable disease” symptoms experienced in the 4 weeks prior to blood collection
All environmental exposures differed significantly between rural and urban households (Table 2). Rural households had higher exposure to livestock, visible excrement around the house, particulate-generating cooking fuel, and car/motorcycle exhaust. Conversely, urban households had higher incomes and a greater proportion had access to running water and flushing toilets.
Table 2. Household characteristicsa.
| Urban | Rural | |
|---|---|---|
| Mean (STD)/% (N) | Mean (STD)/% (N) | |
| N | 1,375 | 2,871 |
| Income, yuan | 40,119.13 (38,723.89) | 31,830.82 (46,296.25)* |
| Exposure to livestock | 8.22 (113) | 33.11 (950)* |
| Toilet in the home | 86.69 (1,192) | 55.63 (1,597)* |
| Access to running water | 80.65 (1,109) | 40.16 (1,153)* |
| Visible excrement | 11.37 (156) | 29.35 (839)* |
| Coal, wood or charcoal cooking fuel | 13.00 (178) | 42.09 (1,200)* |
| High prevalence of cars and motorcyclesb | 23.35 (321) | 64.16 (1,842)* |
p<0.05 t-test and Chi-square tests for difference between urban and rural households
Values represent percent of households with the exposure
Household is in a community with >median density of cars and motorcycles.
Prevalence of Inflammation
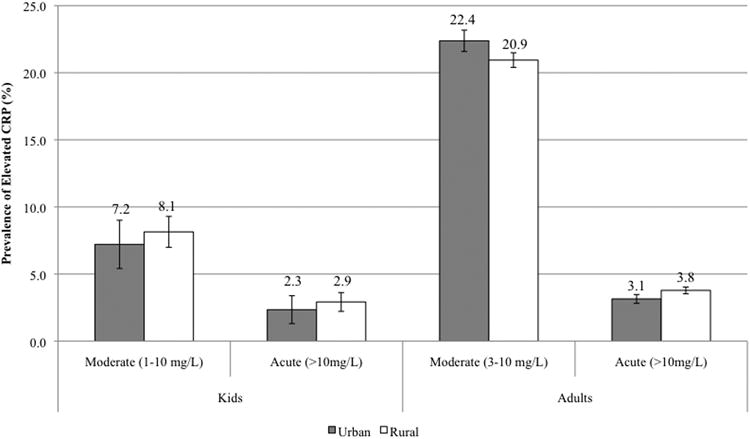
The prevalence of moderate (1-10mg/L) and acute (>10 mg/L) CRP levels in children was 7.89% and 2.77%, respectively. Adults exhibited a higher prevalence of moderate inflammation (CRP 3-10mg/L) at 21.42% and acute inflammation (CRP>10mg/L) at 3.58%. The prevalence of acute CRP was significantly higher in adults with low household income compared to medium/high income (4.11% vs 3.18%; p<0.05); no other significant differences in prevalence between income groups were found (Figure 1). Similarly, there were no statistically significant differences in the prevalence of moderate and acute CRP by urban/rural residence in either children or adults (Figure 2).
Fig 1. Prevalence of moderate and acute elevations in CRP in children, adolescents and adults by income; *p<0.05 for χ2 test of group difference.
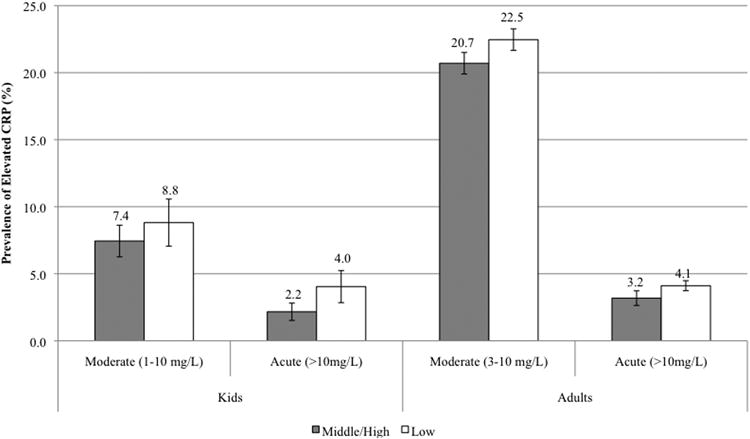
Fig 2. Prevalence of moderate and acute elevations in CRP in children, adolescents and adults by urban/rural residence.
Pathogenic predictors of inflammation
We found statistically significant associations between individuals' illness histories and elevated CRP in the initial individual models (Table 3). Few symptoms were associated with the risk of elevated CRP in children; however, children and adolescents with a history of diarrhea in the past month were more likely to have moderately elevated CRP levels than normal CRP (RRR: 3.52; 95% CI 1.01, 12.27).
Table 3. Association between elevated CRP and illness symptoms in the past 4 weeks a.
| Children and Adolescents | Adults | |||
|---|---|---|---|---|
| Moderate | Acute | Moderate | Acute | |
| CRP 1-10 mg/L RRR [95% CI] |
CRP >10 mg/L RRR [95% CI] |
CRP 3-10mg/L RRR [95% CI] |
CRP >10 mg/L RRR [95% CI] |
|
| Any symptoms | 1.09 [0.59,2.04] |
0.82 [0.19, 3.64] |
1.34 [1.18,1.53] |
1.70 [1.31, 2.20] |
| Any infectious symptomsa | 1.17 [0.61, 2.23] |
0.96 [0.22, 4.26] |
1.27 [1.08, 1.50] |
1.87 [1.38, 2.54] |
| Fever/cough/sore throat | 0.82 [0.38, 1.75] |
1.08 [0.24, 4.75] |
1.26 [1.05,1.52] |
1.79 [1.27, 2.52] |
| Diarrhea |
3.52 [1.01, 12.27] |
--- |
1.63 [1.10, 2.43] |
1.46 [0.63,3.34] |
| Any non-communicable symptomsb | --- | --- |
1.61 [1.33, 1.96] |
1.43 [0.98, 2.11] |
| Heart problem | --- | --- |
1.67 [1.18,2.36] |
1.02 [0.46,2.27] |
| Asthma | --- | --- | 1.72 [0.99, 2.96] |
3.95 [1.99,7.84] |
Relative risk ratio represents the likelihood of moderately or acutely elevated CRP (compared to non-elevated) based on symptom experience, controlling for age strata and sex from individual multinomial logistic models for each symptom/symptom category. Estimates and standard errors are corrected for clustering by household. Significant results (p<0.05) are bolded.
Includes fever/cough/sore throat, diarrhea and “other infectious disease” symptoms experienced in the 4 weeks prior to blood collection
Includes heart problems and “other non-communicable disease” symptoms experienced in the 4 weeks prior to blood collection
The associations between symptom history and inflammation were more extensive in adults in whom both infectious and non-communicable illness symptoms were associated with an increased likelihood of having elevated CRP. Experiencing any of the reported symptoms, with the exception of asthma symptoms which did not reach the p<0.05 level of significance, was associated with a greater likelihood of having moderately elevated CRP. Having experienced any illness symptoms, any infectious symptoms, fever/cough/sore throat, or asthma in the past month was associated with an increased likelihood of acutely elevated CRP. Thus, in adults, both infectious and non-communicable symptoms were associated with moderately elevated CRP while infectious symptoms and asthma were associated with acutely elevated CRP.
Household environmental exposures were significantly associated with the risk of elevated CRP in adults but not children in initial models (Table 4). In children and adolescents, only exposure to particulate-producing cooking fuels was associated with a higher risk of moderately elevated CRP, but this association did not reach a p<0.05 level of significance (RRR: 1.47, 95% CI 0.98-2.20, p=0.06). In adults, exposure to a range of pathogenic factors, including not having a toilet in the home, having excrement visible around the home, and living in an area with a high prevalence of automobiles and motorcycles, were significantly associated with a lower likelihood of moderately elevated CRP. Several exposures had contrasting effects on the risk of moderate versus acute inflammation, though not all reached significance at the p<0.05 level (p<0.10 for all models). Exposure to livestock, not having a toilet in the home, or having excrement visible around the home were associated with a lower likelihood of having moderately elevated CRP and a greater likelihood of having acutely elevated CRP.
Table 4. Associations between elevated CRP and household environmental characteristics.
| Children and Adolescents | Adults | |||
|---|---|---|---|---|
| Moderate | Acute | Moderate | Acute | |
| CRP 1-10 mg/L RRR [95% CI]a |
CRP >10 mg/L RRR[95% CI] |
CRP 3-10 mg/L RRR [95% CI] |
CRP >10 mg/L RRR [95% CI] |
|
| Exposure to livestock | 1.31 [0.87,1.98] |
1.48 [0.62, 3.50] |
0.88 [0.77, 1.00] |
1.28 [1.00, 1.66] |
| No toilet in the home | 0.99 [0.66, 1.48] |
0.56 [0.23, 1.36] |
0.83 [0.75, 0.93] |
1.23 [0.97, 1.55] |
| No access to running water | 0.86 [0.57, 1.31] |
0.55 [0.21, 1.45] |
0.91 [0.81, 1.02] |
1.11 [0.87,1.42] |
| Visible excrement | 1.16 [0.75,1.77] |
1.20 [0.48, 3.02] |
0.85 [0.74, 0.97] |
1.28 [0.98.1.66] |
| Coal,wood or charcoal cooking fuel | 1.47 [0.98, 2.20] |
1.37 [0.58, 3.23] |
0.89 [0.80, 1.00] |
1.19 [0.94, 1.52] |
| High prevalence of cars and motorcycles | 0.89 [0.59, 1.35] |
1.59 [0.62, 4.03] |
0.83 [0.74, 0.92] |
0.95 [0.76, 1.20] |
RRR (relative risk ratio) represents the likelihood of moderately or acutely elevated CRP (compared to non-elevated) based on exposure to household conditions, controlling for age strata and sex from individual multinomial logistic models for each exposure. Estimates and standard errors are corrected for clustering by household. Significant results (p<0.05) are bolded.
Obesogenic predictors of inflammation: In both children and adults, overweight and high waist circumference were associated with a significantly higher risk of moderately elevated CRP in initial models (Table 5). Overweight adults were also at greater risk of having acutely elevated CRP. Neither diet nor physical activity were significantly associated with risk of inflammation in children or adults at the p<0.05 level.
Table 5. Associations between elevated CRP and obesogenic measures.
| Children and Adolescents | Adults | |||
|---|---|---|---|---|
| Moderate | Acute | Moderate | Acute | |
| CRP 1-10 mg/L RRR [95% CI] |
CRP >10 mg/L RRR [95% CI] |
CRP 3-10 mg/L RRR [95% CI] |
CRP >10 mg/L RRR [95% CI] |
|
| Overweight |
3.00 [1.77, 5.06] |
1.00 [0.23, 4.43] |
2.21 [1.97,2.48] |
1.49 [1.16, 1.91] |
| High Waist Circumference |
2.58 [1.52, 4.36] |
1.51 [0.45, 5.05] |
2.20 [1.95,2.46] |
1.26 [0.98, 1.62] |
| Smoking | -- | -- | 0.97 [0.84, 1.12] |
1.14 [0.84, 1.54] |
| High-fat intake | 1.11 [0.74, 1.66] |
1.05 [0.43, 2.52] |
1.12 [1.00, 1.25] |
0.89 [0.71, 1.38] |
| Low leisure PAL | 1.07 [0.66, 1.75] |
0.60 [0.24, 1.53] |
1.00 [0.66, 1.75] |
0.60 [0.24, 1.53] |
RRR (relative risk ratio) represents the likelihood of moderately or acutely elevated CRP (compared to non-elevated) based on characteristic, controlling for age strata and sex from individual multinomial logistic models for each characteristic. Estimates and standard errors are corrected for clustering by household. Significant results (p<0.05) are bolded.
Overall models
Model selection identified predictors remaining independently associated with moderate and acute elevations in CRP in children and adults when controlling for simultaneous exposures to a number of pathogenic and obesogenic factors present in the environment (Table 6). In children, overweight, high waist circumference, and exposure to particulate-forming cooking fuels had p-values <0.10 and were retained in the combined multinomial models predicting moderate and acute inflammation. Overweight was the only variable significantly associated with CRP levels in these full models at the p<0.05 level; overweight children and adolescents were two times more likely to have moderate inflammation, controlling for all other exposures remaining in the model and age, sex, and clustering by household. No pathogenic or obesogenic exposures significantly predicted acutely elevated CRP in children and adolescents.
Table 6. Independent associations between pathogenic and obesogenic factors and elevated CRPa.
| Children and Adolescents | Adults | ||||
|---|---|---|---|---|---|
| Moderate | Acute | Moderate | Acute | ||
| Selected variables | CRP 1-10 mg/L RRR [95% CI] |
CRP >10 mg/L RRR [95% CI] |
Selected variables | CRP 3-10mg/L RRR [95% CI] |
CRP >10 mg/L RRR [95% CI] |
| Overweight |
2.10 [1.13, 3.89] |
0.66 [0.11, 4.07] |
Any ID symptoms |
1.28 [1.08, 1.52] |
1.87 [1.35, 2.56] |
| High Waist Circ | 1.69 [0.91, 3.13] |
2.20 [0.11, 8.31] |
Any NCD symptoms |
1.37 [1.12, 1.69] |
1.42 [0.96, 2.10] |
| Coal,wood or charcoal cooking fuel | 1.50 [0.98, 2.29] |
1.46 [0.61, 3.47] |
Overweight |
1.66 [1.45, 1.89] |
1.40 [1.04, 1.88] |
| High waist circ |
1.63 [1.42, 1.87] |
1.01 [0.76, 1.36] |
|||
| No toilet in the home |
0.86 [0.77, 0.97] |
1.29 [1.00, 1.65] |
|||
| High prevalence of cars and motorcycles |
0.87 [0.77, 0.98] |
0.88 [0.69, 1.12] |
|||
Results from backward model selection initially including all pathogenic and obesogenic factors with factors with p>0.10 retained in multinomial logistic regression models, adjusted for age stratum and gender. Estimates and standard errors are corrected for clustering by household. Significant results (p<0.05) are bolded.
In adults, a number of pathogenic and obesogenic exposures were retained in the final model, including history of any infectious symptoms, any NCD symptoms, overweight, high waist circumference, having no toilet in the home, and living in a community with a high prevalence of cars and motorcycles. Having experienced infectious and non-communicable illness symptoms, being overweight and having high waist circumference were significantly independently associated with a greater risk of moderate CRP elevation, while having no toilet in the home or living in a community with a high prevalence of cars and motorcycles were independently associated with a reduced likelihood of having of moderately elevated CRP. A history of infectious illness symptoms, overweight, and not having a toilet in the home were associated with a greater risk of acutely elevated CRP.
Discussion
Previous research on the inflammatory response has tended to focus on acute inflammation and the activation of the immune system in response to pathogens in developing countries and chronic inflammation and the development of cardiometabolic disease in developed countries. Recent calls have stressed the importance of a more integrated approach for understanding the range of variation in inflammatory processes and their consequences for the global burden of disease (McDade 2012). Consequently, we examined the pathogenic and obesogenic correlates of both moderate and acute levels of CRP in a country currently facing a dual burden of infectious and chronic disease. We found appreciable levels of moderate and acutely elevated CRP at all ages. Approximately 8% of children had levels of CRP indicative of moderate inflammation, a prevalence comparable to the 10% seen in American children (Ford et al. 2003). The prevalence of moderate inflammation was higher in adults-- 21% had CRP between 3-10mg/L-- and intermediate between reported prevalence for Filipino young adults, 7.3%, (McDade et al. 2009b) and American adults, 29% (Woloshin and Schwartz 2005). The prevalence of acute inflammation was around 3% in children and adults, a prevalence similar to children in United States, <5% (Ford et al. 2003) or adults in the Philippines, 3.3-4.5% (McDade et al. 2008; McDade et al. 2009b).
Similar to previous research in populations undergoing the nutrition transition (McDade et al. 2008; McDade et al. 2009b; Vahdat et al. 2012), we found that markers of pathogenic and obesogenic environments were individually and independently associated with inflammation in our sample of Chinese children, adolescents and adults. However, contrary to our theoretical predictions and some previous research (McDade et al. 2005), obesogenic factors had the strongest individual associations with inflammation in children and adults alike based on their estimated effect sizes. Overweight remained significantly associated with CRP when other obesogenic and pathogenic measures were included in the final combined models, suggesting that overweight is an important proximate factor contributing to inflammation. Further, overweight was a significant predictor of moderate inflammation in even the youngest children (ages 7-12.99), with no significant age-interaction found in the association between overweight or waist circumference and elevated CRP (data not shown). These results are similar to those seen in American children and adolescents (Cook et al. 2000; Dowd et al. 2010), where markers of adiposity were the strongest risk factors for inflammation at young ages, and may indicate that increased adiposity is the predominant risk factor for Chinese children growing up in an increasingly obesogenic context (Shan et al. 2010).
Conversely, we found few statistically significant associations between socioeconomic status, residence or markers of environmental pathogenicity and moderate CRP and no significant predictors of acute CRP in children and adolescents. Only a history of diarrhea in the past month and exposure to particulate-producing cooking fuel were at least marginally associated with moderate elevations in CRP in children. This finding of an association between local air quality and inflammation, while crude, is important given the documented links between chronic exposure to poor air quality in childhood to moderate inflammation and later heart disease (Kelishadi et al. 2009; Shima 2007) and recent work documenting the pernicious effect of coalburning on lifespans in China (Chen et al. 2013). Due to this potential for long-term health effects, we additionally tested whether urban/rural residence modified the association between cooking fuel exposure and inflammation (results not shown). We found no strong evidence for effect modification, suggesting that individual exposure may be more important than residence in predicting inflammation. Rural children are more likely to be exposed to particulate-generating cooking fuel, however, and may be at higher risk for long-term health impacts. The effect of exposure to polluting cooking fuels was attenuated in the final combined model when obesogenic risk factors were included; nonetheless, the persistently large relative risk ratio for measures of local air quality indicates that this factor and its potential interaction with residence and overweight merits further investigation.
The associations between pathogenic and obesogenic predictors and moderate and acute inflammation were more extensive in adults. Similar to previous research (McDade et al. 2008; McDade et al. 2009b; Vahdat et al. 2012), both pathogen burden, as measured through illness history, and measures of adiposity, overweight and high waist circumference, were independently associated with a greater likelihood of moderate elevations in CRP. All infectious and noncommunicable disease symptoms were associated with moderate inflammation while only infectious symptoms and asthma were associated with acute inflammation. The wide recall period (4 weeks) and the varying lengths of time between illness episodes and the biomarker measurement may underlie these patterns; nonetheless, these results suggest that inflammatory responses to illness are frequent. The presence of multiple chronic infections has been hypothesized to contribute to a systemic pro-inflammatory environment that, at least in developed countries, may be further exacerbated by overweight and non-communicable disease processes to contribute to earlier morbidity and mortality (Crimmins and Finch 2006; Dowd et al. 2009). Our findings that overweight and infectious disease symptoms are independently associated with both moderate and acute elevations in CRP provide preliminary support that the dual burden of infectious disease exposure and overweight may place individuals at risk for the development of inflammation and cardiovascular disease.
Pathogen burden, measured through illness history, and environmental exposures to pathogens, measured through household sanitation, however, had contrasting associations with adult CRP levels. Markers of environmental pathogenicity, specifically exposure to livestock, not having a toilet in the home, having excrement visible around the home, and living in a community with a high prevalence of car exhaust, were associated with a lower risk of moderate inflammation. Conversely, these markers showed a positive association with the likelihood of having CRP >10mg/L, though not all reached significance at the p<0.05 level. Previous studies have not specifically focused on the association between measures of environmental pathogenicity and CRP levels >10 mg/L; however, our findings that measures of environmental pathogen exposures are associated with a reduced risk of moderate inflammation contrast those from other developing country settings despite our use of similar environmental measures. Among both young adults and middle-aged women in the Philippines, greater environmental pathogenicity was associated with higher risk of having CRP levels from 3-10mg/L (McDade et al. 2008; McDade et al. 2009b). These contrasting results, which remain significant even when controlling for illness history and overweight/high waist circumference in the final combined model, may reflect differences in the distribution of the specific factors associated with environmental hygiene and sanitation between China and Cebu, such as the proportion of participants with access to running water or with a toilet in the home in urban vs. rural areas. Further research would be needed to more fully capture individuals' exposures to varying hygiene and sanitation structures and practices in the two settings.
This finding that pathogen exposure in adults is oppositely associated with moderate and acute inflammation is particularly interesting in light of theoretical predictions of the hygiene hypothesis and ecological immunology (McDade 2012; Musso et al. 2010). Exposure to less hygienic environments in early life has been proposed to regulate long-term immune function by increasing the frequency of exposure to a multitude of pathogens leading to bouts of acute inflammation. This early acute activation may promote development of more competent regulatory pathways that minimize the chronic inflammatory response (McDade 2012; Musso et al. 2010). Previous studies looking at early life pathogen exposure have found conflicting support for this hypothesis with some studies finding childhood illness history associated with elevated CRP in adulthood (Zhu et al. 2000) and others finding an association between childhood pathogen exposures and lower adult CRP (McDade et al. 2009a). We found that adults currently exposed to environmental pathogens are less likely to have moderate inflammation. In so far as these exposures cluster in adults living in rural households, who may also be less affected by the nutrition transition and living more traditional lifestyles both currently and during their childhoods, these results provide preliminary support for the hypothesis that early life exposures to pathogens may be protective against later moderate inflammation, a possible marker of chronic inflammation. Whether this effect remains protective with continued exposure to pathogens, particularly in concert with increasing levels of adiposity, is less clear. Although not having a toilet in the home remained independently protective against moderate inflammation in the combined models, recent illness history and overweight were associated with a greater likelihood of both moderate and acute inflammation in adulthood and measures of environmental pathogenicity and overweight were associated with a greater likelihood of acute inflammation. These results suggest that as rural populations become increasingly exposed to obesogenic environments, inflammation may contribute to an even greater burden of disease.
The CHNS provides a wealth of individual and household measures collected across a wide range of ages and socioeconomic and environmental contexts. Consequently, we were able to assess the impact of a number of pathogenic and obesogenic markers on both acute and moderate inflammation across the life course during a time of rapid social and environmental change. Along with these strengths, our study has several limitations. First, we relied on self-reported illness symptoms over the past 4 weeks as one of our measures of pathogen burden. Self-report is subject to recall bias and under-reporting among sicker individuals (Panter-Brick et al. 2001); however, previous studies have shown that reported symptoms are significantly associated with biomarkers of infection in children (Filteau et al. 1995; Wander et al. 2012) and adults (Nazmi et al. 2010a; Zhu et al. 2000). More importantly, we are limited to a CRP measurement at a single time point, which may introduce error into our assessment of moderate vs. acute inflammation, since we cannot definitively state whether a CRP level of 5, for example, represents chronic elevations in baseline inflammation or recovery from a recent illness. Serial measures are necessary to distinguish between these possibilities and we have attempted to be conservative in our use of the term “moderate elevations” versus “chronic elevation.” Finally, our choice of cut-points for moderate and acute inflammation are derived from predominantly Western populations (Pearson et al. 2004; Skinner et al. 2010) and the appropriateness of these markers for moderate and acute inflammation for other populations has been questioned (Wander et al. 2012). We conducted sensitivity analyses (not shown) with alternate cut-points, a value of >5 mg/L for acute inflammation in children and adolescents based on work by McDade and colleagues (2005) in Bolivia and a value of >2 mg/L for moderate inflammation in adults based on work by Zhao and colleagues (2010) showing that only 15% of Chinese adults had CRP levels >2.06 mg/L. Results from this sensitivity testing did not differ from those presented with exception of adiposity measures in children. When a cut-point of >5mg/L was used as measure of acute inflammation, overweight and high waist circumference became significant predictors of both moderate and acute CRP levels in the individual and combined models, providing stronger evidence that adiposity is the main contributor to inflammation in Chinese children and adolescents.
Conclusion
Our findings provide evidence that both pathogenic and obesogenic factors are independently associated with acute and chronic inflammation in children, adolescents, and adults in a context of rapid environmental and socioeconomic change. Contrary to our expectations, pathogenic factors were more pronounced in adults than in children and adolescents, for whom measures of adiposity were more salient risk factors. These results suggest that Chinese children, much like their American counterparts, are growing up in a post-nutrition transition society where early life weight gain may be a dominant factor shaping long-term morbidity and mortality risk.
In adults, the situation is more complex. Along with overweight and visceral adiposity, illness history and environmental pathogenicity had significant, though contrasting, associations with CRP levels. Overweight and current pathogen burden were independent predictors of the likelihood of both moderate and acute inflammation; however, concurrent exposures to environmental pathogens remained protective against moderate inflammation even after controlling for adiposity. Further longitudinal research is needed to understand the effect of exposure to changing disease burdens and nutritional environments on the trajectory of inflammation across the life course and its role in the development of cardiometabolic disease. Nonetheless, the appreciable levels of inflammation seen at all ages and the association between adiposity and inflammation in even young children indicate that inflammation is and will increasingly become an important health concern. These results highlight the importance of examining the dynamics of both moderate and acute inflammation in societies undergoing nutritional and epidemiological transitions and, perhaps even more importantly, understanding age-based differences in vulnerability to these changing physical and social environments in the development of inflammation.
Acknowledgments
This work was supported by NIH NICHD (K01 HD071948-01). PGL acknowledges support from NHLBI (R01HL108427). We thank the Institute of Nutrition and Food Safety, China Center for Disease Control and Prevention, the Carolina Population Center at the University of North Carolina at Chapel Hill, the NIH (R01-HD30880, DK056350, and R01-HD38700) and the Fogarty International Center, NIH for financial support for the CHNS data collection and analysis files from 1989 to 2011 and future surveys, and the China-Japan Friendship Hospital, Ministry of Health for support for CHNS 2009. We thank the Carolina Population Center at the University of North Carolina at Chapel Hill (5 R24 HD050924) for general support.
Grant information: This analysis and write-up was supported by NIH NICHD (K01 HD071948-01) to ALT. Data collection and analysis files for the CHNS was supported by the NIH (R01-HD30880, DK056350, and R01-HD38700), the Institute of Nutrition and Food Safety, China Center for Disease Control and Prevention, the Fogarty International Center, NIH and the China-Japan Friendship Hospital, Ministry of Health. PGL is supported by NHLBI (R01HL108427). The Carolina Population Center (5 R24 HD050924) provided general support.
Footnotes
The authors declare that they have no conflict of interest.
References
- Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O'Brien WL, Bassett DR, Jr, Schmitz KH, Emplaincourt PO, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- Alberti K, Zimmet P, Shaw J. Metabolic syndrome, A new worldwide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- Chen Y, Ebenstein A, Greenstone M, Li H. Evidence on the impact of sustained exposure to air pollution on life expectancy from China's Huai River policy. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1300018110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Joseph L, Pilote L. Obesity and C-reactive protein in various populations: a systematic review and meta-analysis. Obes Rev. 2013;14:232–244. doi: 10.1111/obr.12003. [DOI] [PubMed] [Google Scholar]
- Cook DG, Mendall MA, Whincup PH, Carey IM, Ballam L, Morris JE, Miller GJ, Strachan DP. C-reactive protein concentration in children: relationship to adiposity and other cardiovascular risk factors. Atherosclerosis. 2000;149:139–150. doi: 10.1016/s0021-9150(99)00312-3. [DOI] [PubMed] [Google Scholar]
- Crimmins EM, Finch CE. Infection, inflammation, height, and longevity. Proc Natl Acad Sci U S A. 2006;103:498–503. doi: 10.1073/pnas.0501470103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBoer MD, Lima AA, Oria RB, Scharf RJ, Moore SR, Luna MA, Guerrant RL. Early childhood growth failure and the developmental origins of adult disease: do enteric infections and malnutrition increase risk for the metabolic syndrome? Nutr Rev. 2012;70:642–653. doi: 10.1111/j.1753-4887.2012.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd JB, Zajacova A, Aiello A. Early origins of health disparities: burden of infection, health, and socioeconomic status in US children. Soc Sci Med. 2009;68:699–707. doi: 10.1016/j.socscimed.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd JB, Zajacova A, Aiello AE. Predictors of inflammation in U.S. children aged 3-16 years. Am J Prev Med. 2010;39:314–320. doi: 10.1016/j.amepre.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du S, Mroz TA, Zhai F, Popkin BM. Rapid income growth adversely affects diet quality in China--particularly for the poor! Soc Sci Med. 2004;59:1505–1515. doi: 10.1016/j.socscimed.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Filteau SM, Morris SS, Raynes JG, Arthur P, Ross DA, Kirkwood BR, Tomkins AM, Gyapong JO. Vitamin A supplementation, morbidity, and serum acute-phase proteins in young Ghanaian children. Am J Clin Nutr. 1995;62:434–438. doi: 10.1093/ajcn/62.2.434. [DOI] [PubMed] [Google Scholar]
- Ford ES, Giles WH, Myers GL, Rifai N, Ridker PM, Mannino DM. C-reactive protein concentration distribution among US children and young adults: findings from the National Health and Nutrition Examination Survey, 1999-2000. Clin Chem. 2003;49:1353–1357. doi: 10.1373/49.8.1353. [DOI] [PubMed] [Google Scholar]
- Gimeno D, Ferrie JE, Elovainio M, Pulkki-Raback L, Keltikangas-Jarvinen L, Eklund C, Hurme M, Lehtimaki T, Marniemi J, Viikari JS, et al. When do social inequalities in C-reactive protein start? A life course perspective from conception to adulthood in the Cardiovascular Risk in Young Finns Study. Int J Epidemiol. 2008;37:290–298. doi: 10.1093/ije/dym244. [DOI] [PubMed] [Google Scholar]
- Gu D, Reynolds K, Wu X, Chen J, Duan X, Reynolds RF, Whelton PK, He J. Prevalence of the metabolic syndrome and overweight among adults in China. The Lancet. 2005;365:1398–1405. doi: 10.1016/S0140-6736(05)66375-1. [DOI] [PubMed] [Google Scholar]
- Gurven M, Kaplan H, Winking J, Eid Rodriguez D, Vasunilashorn S, Kim JK, Finch C, Crimmins E. Inflammation and infection do not promote arterial aging and cardiovascular disease risk factors among lean horticulturalists. PLoS One. 2009;4:e6590. doi: 10.1371/journal.pone.0006590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Gu D, Wu X, Reynolds K, Duan X, Yao C, Wang J, Chen CS, Chen J, Wildman RP. Major causes of death among men and women in China. N Engl J Med. 2005;353:1124–1134. doi: 10.1056/NEJMsa050467. [DOI] [PubMed] [Google Scholar]
- Jones-Smith JC, Popkin BM. Understanding community context and adult health changes in China: development of an urbanicity scale. Soc Sci Med. 2010;71:1436–1446. doi: 10.1016/j.socscimed.2010.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelishadi R, Mirghaffari N, Poursafa P, Gidding SS. Lifestyle and environmental factors associated with inflammation, oxidative stress and insulin resistance in children. Atherosclerosis. 2009;203:311–319. doi: 10.1016/j.atherosclerosis.2008.06.022. [DOI] [PubMed] [Google Scholar]
- Kushner I, Rzewnicki D, Samols D. What does minor elevation of C-reactive protein signify? Am J Med. 2006;119:166 e117–128. doi: 10.1016/j.amjmed.2005.06.057. [DOI] [PubMed] [Google Scholar]
- Li X, Wang C, Zhang G, Xiao L, Dixon J. Urbanisation and human health in China: spatial features and a systemic perspective. Env Sci Poll Res. 2012;19:1375–1384. doi: 10.1007/s11356-011-0718-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma LJC. Urban transformation in China, 1949-2000: a review and research agenda. Env Plan A. 2002;34:1545–1570. [Google Scholar]
- McDade TW. Early environments and the ecology of inflammation. Proc Natl Acad Sci U S A. 2012;109(2):17281–17288. doi: 10.1073/pnas.1202244109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade TW, Leonard WR, Burhop J, Reyes-Garcia V, Vadez V, Huanca T, Godoy RA. Predictors of C-reactive protein in Tsimane' 2 to 15 year-olds in lowland Bolivia. Am J Phys Anthropol. 2005;128:906–913. doi: 10.1002/ajpa.20222. [DOI] [PubMed] [Google Scholar]
- McDade TW, Rutherford JN, Adair L, Kuzawa C. Adiposity and pathogen exposure predict C-reactive protein in Filipino women. J Nutr. 2008;138:2442–2447. doi: 10.3945/jn.108.092700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade TW, Rutherford J, Adair L, Kuzawa CW. Early origins of inflammation: microbial exposures in infancy predict lower levels of C-reactive protein in adulthood. Proc Biol Sci. 2009a;277:1129–1137. doi: 10.1098/rspb.2009.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade TW, Rutherford JN, Adair L, Kuzawa C. Population differences in associations between C-reactive protein concentration and adiposity: comparison of young adults in the Philippines and the United States. Am J Clin Nutr. 2009b;89:1237–1245. doi: 10.3945/ajcn.2008.27080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monda KL, Adair LS, Zhai F, Popkin BM. Longitudinal relationships between occupational and domestic physical activity patterns and body weight in China. Eur J Clin Nutr. 2008;62:1318–1325. doi: 10.1038/sj.ejcn.1602849. [DOI] [PubMed] [Google Scholar]
- Musso G, Gambino R, Cassader M. Obesity, Diabetes, and Gut Microbiota The hygiene hypothesis expanded? Diabetes Care. 2010;33:2277–2284. doi: 10.2337/dc10-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazmi A, Diez-Roux A, Jenny N, Tsai M, Szklo M, Aiello A. The influence of persistent pathogens on circulating levels of inflammatory markers: a cross-sectional analysis from the Multi-Ethnic Study of Atherosclerosis. BMC Public Health. 2010a;10:706. doi: 10.1186/1471-2458-10-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazmi A, Oliveira IO, Horta BL, Gigante DP, Victora CG. Lifecourse socioeconomic trajectories and C-reactive protein levels in young adults: findings from a Brazilian birth cohort. Soc Sci Med. 2010b;70:1229–1236. doi: 10.1016/j.socscimed.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng SW, Norton EC, Popkin BM. Why have physical activity levels declined among Chinese adults? Findings from the 1991-2006 China Health and Nutrition Surveys. Soc Sci Med. 2009;68:1305–1314. doi: 10.1016/j.socscimed.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng SW, Popkin BM. Time use and physical activity: a shift away from movement across the globe. Obes Rev. 2012;13:659–680. doi: 10.1111/j.1467-789X.2011.00982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen XM, Lane J, Smith BR, Nguyen NT. Changes in inflammatory biomarkers across weight classes in a representative US population: a link between obesity and inflammation. J Gastrointest Surg. 2009;13:1205–1212. doi: 10.1007/s11605-009-0904-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panter-Brick C, Lunn PG, Baker R, Todd A. Elevated acute-phase protein in stunted Nepali children reporting low morbidity: different rural and urban profiles. Br J Nutr. 2001;85:125–131. doi: 10.1079/bjn2000225. [DOI] [PubMed] [Google Scholar]
- Park HS, Park JY, Yu R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-alpha and IL-6. Diabetes Res Clin Pract. 2005;69:29–35. doi: 10.1016/j.diabres.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Hong Y, Smith SC., Jr CDC/AHA Workshop on Markers of Inflammation and Cardiovascular Disease: Application to Clinical and Public Health Practice: overview. Circulation. 2004;110:e543–544. doi: 10.1161/01.CIR.0000148979.11121.6B. [DOI] [PubMed] [Google Scholar]
- Popkin BM, Du S, Zhai F, Zhang B. Cohort Profile: The China Health and Nutrition Survey--monitoring and understanding socio-economic and health change in China, 1989-2011. Int J Epidemiol. 2010;39:1435–1440. doi: 10.1093/ije/dyp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popkin BM, Gordon-Larsen P. The nutrition transition: worldwide obesity dynamics and their determinants. Int J Obes. 2004;28:S2–S9. doi: 10.1038/sj.ijo.0802804. [DOI] [PubMed] [Google Scholar]
- Popkin BM, Kim S, Rusev E, Du S, Zizza C. Measuring the full economic costs of diet, physical activity and obesity related chronic diseases. Obes Rev. 2006;7:271–293. doi: 10.1111/j.1467-789X.2006.00230.x. [DOI] [PubMed] [Google Scholar]
- Popkin BM, Lu B, Zhai F. Understanding the nutrition transition: measuring rapid dietary changes in transitional countries. Pub Health Nutr. 2002;5:947–954. doi: 10.1079/PHN2002370. [DOI] [PubMed] [Google Scholar]
- Schaffler A, Muller-Ladner U, Scholmerich J, Buchler C. Role of adipose tissue as an inflammatory organ in human diseases. Endocr Rev. 2006;27:449–467. doi: 10.1210/er.2005-0022. [DOI] [PubMed] [Google Scholar]
- Shan XY, Xi B, Cheng H, Hou DQ, Wang Y, Mi J. Prevalence and behavioral risk factors of overweight and obesity among children aged 2-18 in Beijing, China. Int J Pediatr Obes. 2010;5:383–389. doi: 10.3109/17477160903572001. [DOI] [PubMed] [Google Scholar]
- Shima M. Air pollution and serum C-reactive protein concentration in children. J Epidemiol. 2007;17:169–176. doi: 10.2188/jea.17.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner AC, Steiner MJ, Henderson FW, Perrin EM. Multiple markers of inflammation and weight status: cross-sectional analyses throughout childhood. Pediatrics. 2010;125:e801–809. doi: 10.1542/peds.2009-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, Moodie ML, Gortmaker SL. The global obesity pandemic: shaped by global drivers and local environments. The Lancet. 2011;378:804–814. doi: 10.1016/S0140-6736(11)60813-1. [DOI] [PubMed] [Google Scholar]
- Vahdat K, Azizi F, Zandi K, Assadi M, Nabipour I. Chronic inflammation is correlated with percentage of body fat independent of the burden of infection. Inflammation. 2012;35:1322–1329. doi: 10.1007/s10753-012-9445-6. [DOI] [PubMed] [Google Scholar]
- Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–2135. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- Wander K, Brindle E, O'Connor KA. Sensitivity and specificity of C-reactive protein and alpha(1) -acid glycoprotein for episodes of acute infection among children in Kilimanjaro, Tanzania. Am J Hum Biol. 2012;24:565–568. doi: 10.1002/ajhb.22261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Du S, Zhai F, Popkin BM. Trends in the distribution of body mass index among Chinese adults, aged 20-45 years (1989-2000) Int J Obes. 2007;31:272–278. doi: 10.1038/sj.ijo.0803416. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhai F, Du S, Popkin B. Dynamic shifts in Chinese eating behaviors. Asia Pac J Clin Nutr. 2008;17:123–130. [PubMed] [Google Scholar]
- Woloshin S, Schwartz LM. Distribution of C-reactive protein values in the United States. N Engl J Med. 2005;352:1611–1613. doi: 10.1056/NEJM200504143521525. [DOI] [PubMed] [Google Scholar]
- Yan S, Li J, Li S, Zhang B, Du S, Gordon-Larsen P, Adair L, Popkin B. The expanding burden of cardiometabolic risk in China: the China Health and Nutrition Survey. Obes Rev. 2012;13:810–821. doi: 10.1111/j.1467-789X.2012.01016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Yu Z, Li H, Franco OH, Liu Y, Lin X. Distributions of C-reactive protein and its association with metabolic syndrome in middle-aged and older Chinese people. J Amer Coll Card. 2007;49:1798–1805. doi: 10.1016/j.jacc.2007.01.065. [DOI] [PubMed] [Google Scholar]
- Yoon KH, Lee JH, Kim JW, Cho JH, Choi YH, Ko SH, Zimmet P, Son HY. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006;368:1681–1688. doi: 10.1016/S0140-6736(06)69703-1. [DOI] [PubMed] [Google Scholar]
- Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104:2746–2753. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- Zhang KH, Song S. Rural-urban migration and urbanization in China: Evidence from time-series and cross-section analysis. China Economic Review. 2003;14:386–400. [Google Scholar]
- Zhai F, Guo X, Popkin BM, Ma L, Wang Q, Yu W, Jin S, Ge K. Evaluation of the 24-hour individual recall method in China. Food Nutr Bull. 1996;17:154–161. [Google Scholar]
- Zhao Y, Wang R, Ma X, Yan X, Zhang Z, He X, He J. Distribution of C-reactive protein and its association with cardiovascular risk factors in a population-based sample of Chinese. Disease markers. 2010;28:333–342. doi: 10.3233/DMA-2010-0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Quyyumi AA, Norman JE, Csako G, Waclawiw MA, Shearer GM, Epstein SE. Effects of total pathogen burden on coronary artery disease risk and C-reactive protein levels. Am J Cardiol. 2000;85:140–146. doi: 10.1016/s0002-9149(99)00653-0. [DOI] [PubMed] [Google Scholar]
- Zhu YG, Ioannidis JP, Li H, Jones KC, Martin FL. Understanding and harnessing the health effects of rapid urbanization in China. Env Sci Tech. 2011;45:5099–5104. doi: 10.1021/es2004254. [DOI] [PubMed] [Google Scholar]


