Abstract
Purpose
In thumb carpometacarpal osteoarthritis, current evidence supports that degenerative, bony remodeling primarily occurs within the trapezium. The pathomechanics involved and the most common sites of wear, however, remain controversial. Quantifying structural bone morphology characteristics with high-resolution computed tomography CT (micro-CT) infer regions of load transmission. Using micro-CT, we investigated whether predominant trabecular patterns exist in arthritic vs. normal trapeziums.
Methods
We performed micro-CT analysis on 13 normal cadaveric trapeziums and 16 Eaton stage III–IV trapeziums. Each specimen was computationally divided into 4 quadrants: volar-ulnar, volar-radial, dorsal-radial, and dorsal-ulnar. Measurements of trabecular bone morphologic parameters included bone volume ratio, connectivity, trabecular number, and trabecular thickness. Using analysis of variance with post hoc Bonferroni/Dunn correction, we compared osteoarthritic and normal specimen quadrant measurements.
Results
No significant difference existed in bone volume fraction between the osteoarthritic and normal specimens. Osteoarthritic trapeziums, however, demonstrated significantly higher trabecular number and connectivity than non-osteoarthritic trapeziums. When collectively comparing the volar-ulnar quadrant of osteoarthritis and normal specimens, this quadrant in both consistently possessed significantly higher bone volume fraction, trabecular number, and connectivity than the dorsal-radial and volar-radial quadrants.
Conclusions
The significantly greater trabecular bone volume, thickness, and connectivity in the volar-ulnar quadrant compared to the dorsal-radial and dorsal-ulnar quadrants provides evidence that the greatest compressive loads at the first carpometacarpal joint occur at the volar-ulnar quadrant of the trapezium, representing a consistently affected region of wear in both normal and arthritic states.
Keywords: abnormal loading, arthritis, bone morphology, thumb CMC joint, trapezium
Introduction
Reconstruction for disabling thumb carpometacarpal (CMC) joint arthritis is the most commonly performed surgical procedure for osteoarthritis (OA) in the adult upper extremity (1). CMC OA affects approximately 20–65% of adults with a greater prevalence in post-menopausal women (2–5). Despite the prevalence of CMC OA, the most common sites of articular wear remain controversial (6–16). Preferential wear of the volar surface of the trapezium, however, is the most common pattern of degeneration reported in the literature (9, 10, 13, 17, 18). Similarly, the mechanics of abnormal loading and wear of the metacarpal upon the trapezium, and the bony remodeling of this process, are postulated (19,20) but poorly understood; few in vivo studies of CMC joint kinematics exist (21,22).
Trabecular structure reflects bone activity: according to Wolff, as loading on a bone increases, the bone compensates by remodeling and increasing its trabecular structure at the loading site (23). Examination of the trabecular morphology in bone adjacent to the joint thus permits indirect study of the pathomechanics of osteoarthritis. Current literature supports noteworthy trabecular remodeling primarily in the trapezium rather than the first metacarpal in CMC joint arthritis (13, 15, 17, 20, 24). Recent studies using high-resolution computed tomography (micro-CT) have correlated changes in trabecular bone morphology with osteoarthritis (20, 25–27). These studies have demonstrated substantial deterioration in the 3-dimensional architecture of cancellous bone, leading to unfavorable alteration in mechanical properties. Accordingly, we hypothesized that micro-CT imaging of arthritic trapeziums compared to normal trapeziums would correlate with wear patterns of thumb CMC joint arthritis. In addition, we hypothesized that micro-CT would demonstrate greater difference in bone volume fraction of the volar region of osteoarthritic trapeziums than the dorsal region, as compared to volar and dorsal regions in normal trapeziums.
Materials and Methods
Specimen Harvest
We harvested a total of 29 trapeziums (13 normal, 16 osteoarthritic) for comparison in this study and carefully protected the articular surfaces during cadaveric or surgical explantation. Approval for this project was granted through the local institutional review board, and the handling of human remains strictly adhered to ethical and practical protocols.
We removed 13 normal trapeziums without evidence of osteoarthritis from 11 embalmed cadavers obtained from our institution’s willed body donation program. Cadaveric trapeziums with evidence of articular wear, presence of osteophytes, or damage from harvesting were excluded. For surgical specimens, we used the Wagner approach to remove intact 16 osteoarthritic trapeziums in 16 subjects who underwent elective reconstruction of symptomatic CMC joint arthritis. Preoperative radiographic evaluation determined that these had Eaton stage III–IV osteoarthritis (16). The entire trapezium was excised with the aid of a 3.5 mm tap used as a joystick to visualize investing soft tissues and assist in ligament detachment. The tap was inserted parallel to the distal articular surface from the radial non-articular surface between the trapezial ridge and dorso-radial tubercle and beneath the abductor pollicis longus tendon and directed towards the second metacarpal.
All specimens were stored in 10% formalin following collection. The mean age of the normal and osteoarthritic trapeziums was 69 (range 43 to 96) and 61 (range 54 to 72), respectively, with 4 female/9 male specimens in the normal group and 10 female/6 male specimens in the osteoarthritic group.
Micro-CT Scanning
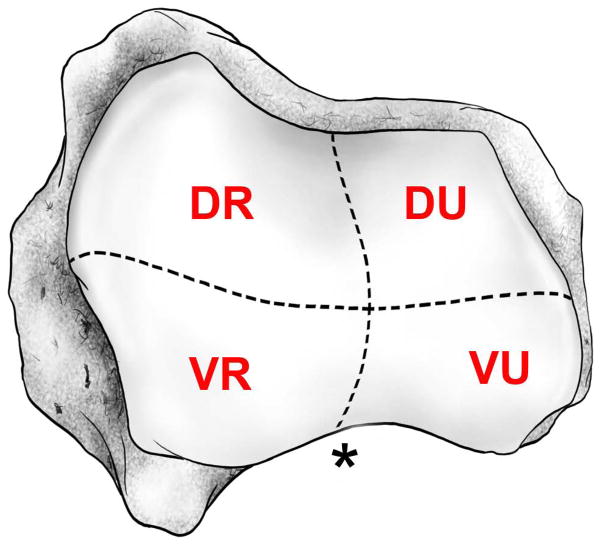
Each trapezium was scanned and reconstructed in a micro-CT system (vivaCT 40, Scanco Medical AG, Wayne, PA) at a resolution of 38 μm isometric voxels (voltage = 55 kVp; current = 145 mA; integration time = 381 msec). Volumetric surface rendering software (Amira, Visage Imaging, San Diego, CA) was used to orient and measure the scanned image of each specimen. Using orthogonal planes, the volume for each specimen was divided into 4 quadrants: dorso-ulnar (DU), dorso-radial (DR), volar-ulnar (VU), and volar-radial (VR) (Figures 1 and 2). These quadrants were delineated according to the XYZ-axis orientation described by Cooney (28) and represent the most common identifying articular landmarks of the thumb CMC joint reported in the literature (11, 13–15, 29–31).
Figure 1.
Schematic drawing of the 4 quadrants of the metacarpal surface of a left trapezium: dorso-ulnar (DU), dorso-radial (DR), volar-ulnar (VU), and volar-radial (VR) (28). The (*) represents the articulating recess of the metacarpal volar beak.
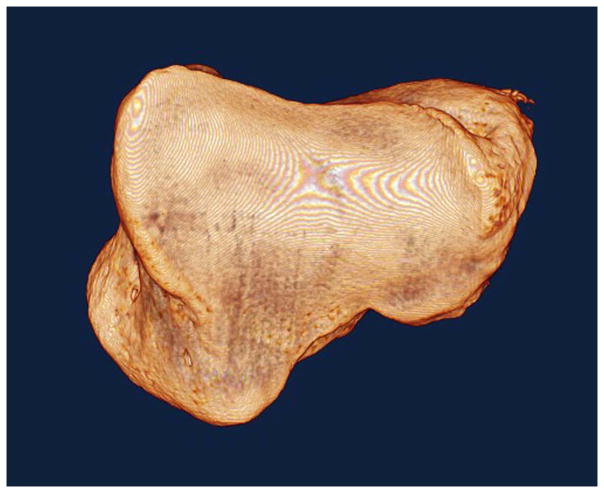
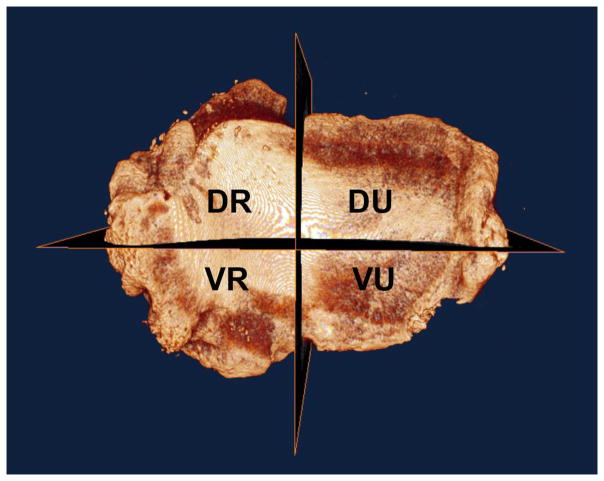
Figure 2.
Micro-CT volumetric renderings of the metacarpal articulating surface of a normal right and an arthritic right trapezium. The normal trapezium (Figure 2a, left): is rendered intact; the arthritic trapezium is divided into 4 quadrants (Figure 2b, right).
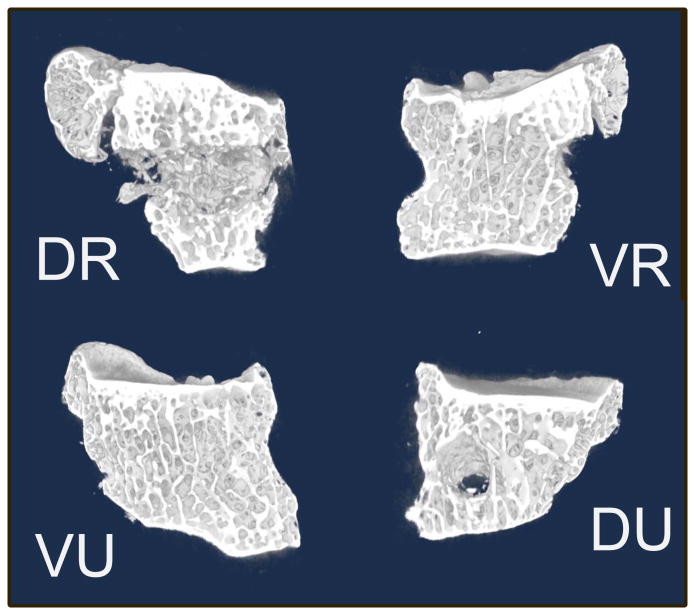
Individual quadrants were then imported into volumetric analysis software (Microview, General Electric, Fairfield, CN) for further quantification. We selected a representative sample of trabecular bone within each quadrant; this rectangular volume excluded subchondral bone, peripheral osteophytes, and the transversely oriented drill hole (Figure 3). Trabecular bone morphologic parameters were obtained for each region of interest as follows: bone volume fraction (BVF), connectivity density (CD), trabecular thickness (Tb.Th), trabecular spacing (Tb.Sp), and trabecular number (Tb.N) (22, 25–27, 32).
Figure 3.
Representative slices in each of the 4 quadrants of an arthritic specimen visualizing trabecular structure. The circular defect in the dorso-ulnar (DU) quadrant is drill artifact.
Morphometric parameters were determined using a direct 3-dimensional technique without assumption of the bone structure type (33). Bone volume fraction was obtained by dividing bone volume (BV) with the total volume (TV) representing apparent bone density (34). Connectivity density was defined as the maximal number of trabeculae that could be cut through within a region of interest without causing the structure to fall apart (35). Trabecular thickness, Tb.Sp, and Tb.N were directly calculated without assumption of a structure model (33).
Statistical Analysis
Statistical analysis using a 2-factor analysis of variance with a Bonferroni/Dunn post hoc test analyzed the differences between the 2 factors investigated (normal/OA and quadrant). A P value of <0.05 was considered to be statistically significant in normal compared to OA trapeziums when comparing entire trabeculae, and P<.0083 for trapezial quadrant comparison.
Results
Bone Volume Fraction
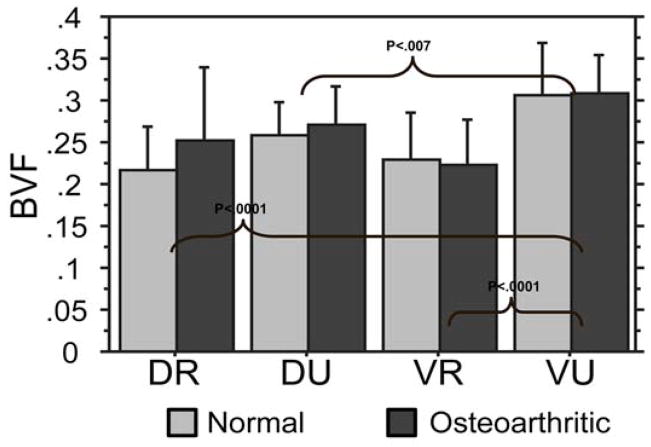
No significant difference existed between normal and osteoarthritic trapeziums in overall bone volume fraction (P=0.312), trabecular spacing (P=0.208), or trabecular thickness (P=0.151). There was also no significant difference in bone volume fraction between osteoarthritic and control groups with respect to individual quadrants. However, in both the osteoarthritic and control groups, analysis of bone morphometric parameters of the 4 quadrants revealed that the VU quadrant bone volume fraction was significantly higher than the VR, DR, and DU (Figure 4). The difference in volume was 35%, 30%, and 16% higher in the VU quadrant compared to the VR, DR, and DU quadrants, respectively.
Figure 4.
Bone volume fraction (BVF): in both osteoarthritic and normal specimens, the VU quadrant possessed the highest BVF relative to the two radial quadrants (DR and VR, P<.001).
Trabecular Spacing
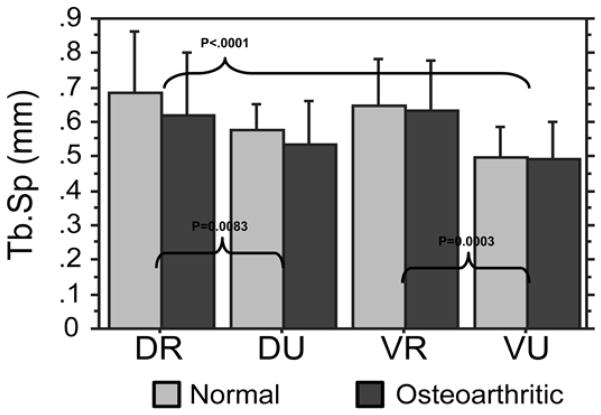
There was no significant difference between osteoarthritic trapeziums and controls with respect to trabecular spacing (P=0.208) in each of the 4 quadrants. The VU quadrant trabecular spacing was significantly lower than the DR and VR quadrants (Figure 5). The DR and VR quadrants displayed 28% and 30% higher trabecular spacing compared to the VU quadrant. The DU trabecular spacing was significantly lower than the DR spacing by 18%.
Figure 5.
Trabecular Spacing (TbSp): the VU quadrant demonstrated lower separation (tighter configuration) than DR and VR quadrants (both P<.001).
Trabecular Number
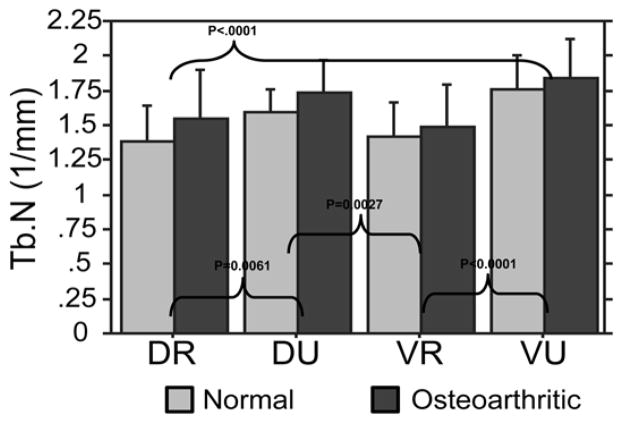
Osteoarthritic trapeziums exhibited greater trabecular number than controls (P=0.025). With respect to quadrants, the VU quadrant trabecular number was significantly higher than the VR and DR (Figure 6). The trabecular number in the VU quadrants was 20% higher than the VR quadrants and 17% higher than the DR quadrants. The DU quadrant trabecular number was also significantly higher than the VR quadrant by 15% and the DR quadrant by 14%.
Figure 6.
Trabecular Number (TbN): both the VU and DU quadrants possessed significantly higher trabecular number than the VR and DR quadrants.
Connectivity
Osteoarthritic trapeziums displayed greater connectivity than controls (P=.0177). With respect to trapezial quadrants, the VU quadrant connectivity (mean=7.11) was significantly higher than the VR (mean=5.17; P=.0008) and DR (mean=5.23; P=.0011). The VU quadrant displayed 38% and 33% more connectivity than the VR and DR quadrants, respectively. The DU quadrant (mean=6.49) was higher than the DR and VR, but these comparisons were not statistically significant (P=.0277 and P=.0207, respectively).
Trabecular Thickness
Osteoarthritic trapeziums exhibited an overall trend of lower trabecular thickness compared to controls, but this was not statistically significant (P=.1505). The VU quadrant (mean=0.158) displayed the highest mean trabecular thickness, and the volar-radial quadrant was the lowest (mean=0.144) (P=.0264; not significant on comparison). There was no significant difference in trabecular thickness between the 4 quadrants: DR (mean=0.148), DU (mean=0.15), VR (mean=0.144), and VU (mean=0.158).
Discussion
This study demonstrated that arthritic trapeziums have statistically increased trabecular number and connectivity but lack significant differences in bone volume fraction and trabecular spacing compared to normal trapeziums. This supports our first hypothesis that volumetric differences can be measured between the 2 specimen types. The mean trabecular thickness was higher for normal than for osteoarthritic trapeziums, but the difference lacked statistical significance. This trend suggests late stage trapezial osteoarthritis involves bone turnover resulting in thinner, less dense trabeculae, which supports other trabecular studies demonstrating an increased bone turnover in later stage osteoarthritis with less structurally sound trabeculae (36, 37). Early stage OA tibial studies, in contrast, demonstrate increased trabecular thickness (27). Reportedly, this increase in trabecular thickness in early OA is associated with decreased mechanical properties of subchondral cancellous bone (27). Although hypothetical, these changes may be secondary to pain-related activity limitation and joint protection that halt robust physiologic structural remodeling (37).
When comparing individual trapezial quadrants, the VU quadrant exhibited the highest bone volume fraction compared to the VR, DU, and DR quadrants. The lowest bone volume fraction was consistently in the VR quadrant. This was true in both osteoarthritic and non-osteoarthritic trapeziums.
Several micro-CT studies have correlated trabecular architectural changes to increased loads associated with joint degeneration (25–27, 32, 36). Controversy exists whether degenerative arthritis is triggered predominantly by biomechanical versus biochemical factors. Our bone morphometric study provides evidence that the greatest physiologic trapeziometacarpal loads occur at the VU quadrant of the trapezium in both normal and osteoarthritic joints. When compared to the surrounding quadrants, the trabeculae in the VU quadrant were characteristically denser, more connected, and thicker, which suggested this was a region exposed to greater compressive forces. These findings partially support our second hypothesis of a higher bone volume fraction in the volar regions and suggest a biochemical trigger of increased bone turnover in pathologic CMC joint osteoarthritis.
Using micro-CT bone morphometric, Nufer and colleagues observed increased bone volume ratio in the ulnar aspect of 15 osteoarthritic and 15 normal trapeziums (20). Their methodology differed as follows: they excluded the peripheral one-third of the bone to remove density artifact from rimming osteophytes, and they divided the trapezium into 3 columns (radial, middle, and ulnar). Using this methodology, they found a significant difference of 42% in bone volume ratio between OA and normal trapeziums. The radial third showed a 67% difference, and the middle and ulnar thirds were 39% and 53% higher in the OA groups, respectively. All these findings were statistically significant. They examined central bone volume in 3 columns oriented dorsovolarly; this does not permit direct comparison to our anatomical quadrant methodology, as reported in the literature (11,13–15,29,30). Thus the density sampling in each study may not be contradictory, as the column approach in Nufer’s study examined uniform central loading absent of rimming osteophytes, whereas our study evaluated articular wear density including the contributing osteophytes and potentially concomitant eccentric load transmission.
Limitations of our study include small sample size and lack of precise matched controls. The osteoarthritic specimens and normal specimens were not controlled in terms of age, sex, handedness, or bone mineral density, thus a variation have existed that minimized differences between osteoarthritic and normal trabecular density. The sample size and representation, however, compares to previously reported data of osteoarthritic and normal bone that similarly lack controlled, matched cohort statistical analysis (20,31,32,37).
Pathomechanics of CMC joint osteoarthritis are not well understood. Functional motion and load analysis of the normal and arthritic CMC has focused on cadaveric biomechanical studies (19,28,38,39) and live subject kinematic analysis, which approximates CMC joint circumduction (21,22,40). Biomechanical analysis as it relates to pathophysiology is similarly limited. Pellegrini and colleagues found no appreciable difference in collagen content between surgically explanted osteoarthritic specimens and elderly arthritic cadaveric specimens (41). They postulated the mechanism for osteoarthritic degeneration likely stems not solely from supra-physiologic shearing forces in the volar trapezial region, but a combination of hormonal, cellular, and inflammatory mediating factors acting at the protective surface lamina of CMC articular cartilage (42). This complements current evidence and theories as it relates to etiology and pathomechanics of osteoarthritis throughout the musculoskeletal system.
We propose the following theory, which unifies the various descriptive and quantifying studies reviewed. The human thumb performs the complex tasks of grasp, pinch, and other precision activity in concert with the fingers. The fingers are positioned medial to the thumb, regardless of the position in space afforded by the wide circumduction of the CMC joint. The unique structure, motor activity, and position of the thumb preferentially favors ulnar rotational loading of the CMC joint in functional tasks, in both normal and arthritic states. Consistent rotational forces in functional tasks create preferential loading in the VU quadrant, corresponding to the increase bone volume in normal and arthritic conditions we observed. This supports theories proposed by Ateshian (11), Edmunds, (8,43) and Pellegrini (10), who proposed a progressive delamination of the protective cartilaginous surface, thus triggering the inflammatory mediators of osteoarthritis.
Our study describes an indirect method of evaluating physiologic stresses of the CMC joint. We anticipate a variety of ongoing and future studies: in vivo kinematic and kinetic CMC joint loading (44), finite element analysis to predict wear patterns of supra-physiologic stresses from functional or abnormal CMC joint motion, and biochemical analysis of inflammatory or hormonal mediators. These investigations will provide direct evidence and enhanced understanding of the pathomechanics contributing to the common affliction of thumb CMC joint osteoarthritis.
Clinical Relevance.
These findings suggest that trapezial trabecular morphology undergoes pathologic alteration. This provides indirect evidence that changes in load transmission occur with thumb carpometacarpal joint arthritis development.
Acknowledgments
Funding Acknowledgement: Funding sources include the Williams Charitable trust, NIH (SBIR)/NBIB R43 EB003067-02A1; OREF/DePuy/RJOS Career Development Award
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Van Heest AE, Kallemeier P. Thumb carpal metacarpal arthritis. J Am Acad Orthop Surg. 2008;16:140–151. doi: 10.5435/00124635-200803000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong AL, Hunter JB, Davis TR. The prevalence of degenerative arthritis of the base of the thumb in post-menopausal women. J Hand Surg. 1994;19B:340–341. doi: 10.1016/0266-7681(94)90085-x. [DOI] [PubMed] [Google Scholar]
- 3.Dahaghin S, Bierma-Zeinstra SM, Ginai AZ, Pols HA, Hazes JM, Koes BW. Prevalence and pattern of radiographic hand osteoarthritis and association with pain and disability (the Rotterdam study) Ann Rheum Dis. 2005;64:682–687. doi: 10.1136/ard.2004.023564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilder FV, Barrett JP, Farina EJ. Joint-specific prevalence of osteoarthritis of the hand. Osteoarthritis Cartilage. 2006;14:953–957. doi: 10.1016/j.joca.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 5.Haara MM, Heliovaara M, Kroger H, Arokoski JP, Manninen P, Karkkainen A, et al. Osteoarthritis in the carpometacarpal joint of the thumb. Prevalence and associations with disability and mortality. J Bone Joint Surg. 2004;86A:1452–1457. doi: 10.2106/00004623-200407000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Eaton RG, Littler JW. Ligament reconstruction for the painful thumb carpometacarpal joint. J Bone Joint Surg. 1973;55A:1655–1666. [PubMed] [Google Scholar]
- 7.Eaton RG, Littler JW. A study of the basal joint of the thumb. Treatment of its disabilities by fusion. J Bone Joint Surg. 1969;51A:661–668. [PubMed] [Google Scholar]
- 8.Edmunds JO. Traumatic dislocations and instability of the trapeziometacarpal joint of the thumb. Hand Clin. 2006;22:365–392. doi: 10.1016/j.hcl.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Pellegrini VD. Osteoarthritis of the trapeziometacarpal joint: the pathophysiology of articular cartilage degeneration. I. Anatomy and pathology of the aging joint. J Hand Surg. 199;16A:967–974. doi: 10.1016/s0363-5023(10)80054-1. [DOI] [PubMed] [Google Scholar]
- 10.Pellegrini VD. The ABJS 2005 Nicolas Andry Award: osteoarthritis and injury at the base of the human thumb: survival of the fittest? Clin Orthop Relat Res. 2005;438:266–276. doi: 10.1097/01.blo.0000176968.28247.5c. [DOI] [PubMed] [Google Scholar]
- 11.Ateshian GA, Ark JW, Rosenwasser MP, Pawluk RJ, Soslowsky LJ, Mow VC. Contact areas in the thumb carpometacarpal joint. J Orthop Res. 1995;13:450–458. doi: 10.1002/jor.1100130320. [DOI] [PubMed] [Google Scholar]
- 12.Kovler M, Lundon K, McKee N, Agur A. The human first carpometacarpal joint: osteoarthritic degeneration and 3-dimensional modeling. J Hand Ther. 2004;17:393–400. [PubMed] [Google Scholar]
- 13.Xu L, Strauch RJ, Ateshian GA, Pawluk RJ, Mow VC, Rosenwasser MP. Topography of the osteoarthritic thumb carpometacarpal joint and its variations with regard to gender, age, site, and osteoarthritic stage. J Hand Surg. 1998;23A:454–464. doi: 10.1016/S0363-5023(05)80463-0. [DOI] [PubMed] [Google Scholar]
- 14.Koff MF, Ugwonali OF, Strauch RJ, Rosenwasser MP, Ateshian GA, Mow VC. Sequential wear patterns of the articular cartilage of the thumb carpometacarpal joint in osteoarthritis. J Hand Surg. 2003;28A:597–604. doi: 10.1016/s0363-5023(03)00145-x. [DOI] [PubMed] [Google Scholar]
- 15.Ateshian GA, Rosenwasser MP, Mow VC. Curvature characteristics and congruence of the thumb carpometacarpal joint: differences between female and male joints. J Biomech. 1992;25:591–607. doi: 10.1016/0021-9290(92)90102-7. [DOI] [PubMed] [Google Scholar]
- 16.Eaton RG, Glickel SZ. Trapeziometacarpal osteoarthritis. Staging as a rationale for treatment. Hand Clin. 1987;3:455–471. [PubMed] [Google Scholar]
- 17.Pelligrini VD. Osteoarthritis of the trapeziometacarpal joint: the pathophysiology of articular cartilage degeneration. II. Articular wear patterns in the osteoarthritic joint. J Hand Surg. 1991;16A:975–982. doi: 10.1016/s0363-5023(10)80055-3. [DOI] [PubMed] [Google Scholar]
- 18.Pellegrini VD, Jr, Olcott CW, Hollenberg G. Contact patterns in the trapeziometacarpal joint: the role of the palmar beak ligament. J Hand Surg. 1993;18A:238–244. doi: 10.1016/0363-5023(93)90354-6. [DOI] [PubMed] [Google Scholar]
- 19.Kuczynski K. Carpometacarpal joint of the human thumb. J Anat. 1974;118:119–126. [PMC free article] [PubMed] [Google Scholar]
- 20.Nufer P, Goldhahn J, Kohler T, Kuhn V, Muller R, Herren DB. Microstructural adaptation in trapeziumsl bone due to subluxation of the thumb. J Orthop Res. 2008;26:208–216. doi: 10.1002/jor.20500. [DOI] [PubMed] [Google Scholar]
- 21.Gehrmann SV, Tang J, Li ZM, Goitz RJ, Windolf J, Kaufmann RA. Motion deficit of the thumb in CMC joint arthritis. J Hand Surg. 2010;35A:1449–1453. doi: 10.1016/j.jhsa.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 22.Miura T, Ohe T, Masuko T. Comparative in vivo kinematic analysis of normal and osteoarthritic trapeziometacarpal joints. J Hand Surg. 2004;29A:252–257. doi: 10.1016/j.jhsa.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Wolff J. Das Gesetz der Transformation der Knochen. Berlin: Hirschwald; 1892. [Google Scholar]
- 24.North ER, Rutledge WM. The trapezium–thumb metacarpal joint: the relationship of joint shape and degenerative joint disease. Hand. 1983;15:201–206. doi: 10.1016/s0072-968x(83)80014-x. [DOI] [PubMed] [Google Scholar]
- 25.Boyd SK, Muller R, Leonard T, Herzog W. Long-term periarticular bone adaptation in a feline knee injury model for post-traumatic experimental osteoarthritis. Osteoarthritis Cartilage. 2005;13:235–242. doi: 10.1016/j.joca.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Boyd SK, Muller R, Zernicke RF. Mechanical and architectural bone adaptation in early stage experimental osteoarthritis. J Bone Miner Res. 2002;17:687–694. doi: 10.1359/jbmr.2002.17.4.687. [DOI] [PubMed] [Google Scholar]
- 27.Ding M, Odgaard A, Hvid I. Changes in the three-dimensional microstructure of human tibial cancellous bone in early osteoarthritis. J Bone Joint Surg. 2003;85B:906–912. [PubMed] [Google Scholar]
- 28.Cooney WP, Lucca MJ, Chao EY, Linscheid RL. The kinesiology of the thumb trapeziometacarpal joint. J Bone Joint Surg. 1981;63A:1371–1381. [PubMed] [Google Scholar]
- 29.Humes D, Jahnich H, Rehm A, Compson JP. The osteology of the trapezium. J Hand Surg. 2004;29B:42–45. doi: 10.1016/j.jhsb.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 30.Momose T, Nakatsuchi Y, Saitoh S. Contact area of the trapeziometacarpal joint. J Hand Surg. 1999;24A:491–495. doi: 10.1053/jhsu.1999.0491. [DOI] [PubMed] [Google Scholar]
- 31.Rivers PA, Rosenwasser MP, Mow VC, Pawluk RJ, Strauch RJ, Sugalski MT, et al. Osteoarthritic changes in the biochemical composition of thumb carpometacarpal joint cartilage and correlation with biomechanical properties. J Hand Surg. 2000;25A:889–898. doi: 10.1053/jhsu.2000.16358. [DOI] [PubMed] [Google Scholar]
- 32.Bobinac D, Spanjol J, Zoricic S, Maric I. Changes in articular cartilage and subchondral bone histomorphometry in osteoarthritic knee joints in humans. Bone. 2003;32:284–290. doi: 10.1016/s8756-3282(02)00982-1. [DOI] [PubMed] [Google Scholar]
- 33.Hildebrand T, Laib A, Muller R, Dequeker J, Ruegsegger P. Direct three-dimensional morphometric analysis of human cancellous bone: microstructural data from spine, femur, iliac crest, and calcaneus. J Bone Miner Res. 1999;14:1167–1174. doi: 10.1359/jbmr.1999.14.7.1167. [DOI] [PubMed] [Google Scholar]
- 34.Muller R, Van Campenhout H, Van Damme B, Van Der Perre G, Dequeker J, Hildebrand T, et al. Morphometric analysis of human bone biopsies: a quantitative structural comparison of histological sections and micro-computed tomography. Bone. 1998;23:59–66. doi: 10.1016/s8756-3282(98)00068-4. [DOI] [PubMed] [Google Scholar]
- 35.Odgaard A, Gundersen HJ. Quantification of connectivity in cancellous bone, with special emphasis on 3-D reconstructions. Bone. 1993;14:173–182. doi: 10.1016/8756-3282(93)90245-6. [DOI] [PubMed] [Google Scholar]
- 36.Dedrick DK, Goldstein SA, Brandt KD, O’Connor BL, Goulet RW, Albrecht M. A longitudinal study of subchondral plate and trabecular bone in cruciate-deficient dogs with osteoarthritis followed up for 54 months. Arthritis Rheum. 1993;36:1460–1467. doi: 10.1002/art.1780361019. [DOI] [PubMed] [Google Scholar]
- 37.Patel V, Issever AS, Burghardt A, Laib A, Ries M, Majumdar S. MicroCT evaluation of normal and osteoarthritic bone structure in human knee specimens. J Orthop Res. 2003;21:6–13. doi: 10.1016/S0736-0266(02)00093-1. [DOI] [PubMed] [Google Scholar]
- 38.Imaeda T, Niebur G, Cooney WP, Linscheid RL, An KN. Kinematics of the normal trapeziometacarpal joint. J Orthop Res. 1994;12:197–204. doi: 10.1002/jor.1100120208. [DOI] [PubMed] [Google Scholar]
- 39.Zancolli EA, Ziadenberg C, Zancolli E. Biomechanics of the trapeziometacarpal joint. Clin Orthop Relat Res. 1987;220:14–26. [PubMed] [Google Scholar]
- 40.Cheze L, Doriot N, Eckert M, Rumelhart C, Comtet JJ. In vivo cinematic study of the trapezometacarpal joint] Chir Main. 2001;20:23–30. doi: 10.1016/s1297-3203(01)00011-7. [DOI] [PubMed] [Google Scholar]
- 41.Pellegrini VD, Jr, Smith RL, Ku CW. Pathobiology of articular cartilage in trapeziometacarpal osteoarthritis. I. Regional biochemical analysis. J Hand Surg. 1994;19A:70–78. doi: 10.1016/0363-5023(94)90226-7. [DOI] [PubMed] [Google Scholar]
- 42.Pellegrini VD, Jr, Smith RL, Ku CW. Pathobiology of articular cartilage in trapeziometacarpal osteoarthritis. II. Surface ultrastructure by scanning electron microscopy. J Hand Surg. 1994;19A:79–85. doi: 10.1016/0363-5023(94)90227-5. [DOI] [PubMed] [Google Scholar]
- 43.Edmunds JO. Current concepts of the anatomy of the thumb trapeziometacarpal joint. J Hand Surg. 2011;36A:170–182. doi: 10.1016/j.jhsa.2010.10.029. [DOI] [PubMed] [Google Scholar]
- 44.Ladd AL, Weiss APC, Crisco JJ, Hagert E, Wolf JM, Glickel SZ. The Thumb CMC Joint: Anatomy, Hormones, and Biomechanics. 2012 AAOS Instructional Course Lectures. in press. [PMC free article] [PubMed] [Google Scholar]