Abstract
Objective
We sought to test the hypothesis that a combined sonographic scoring system (CSTI) that incorporates features of the biophysical profile (BPP) and multi-vessel Doppler evaluation improves prediction of adverse outcomes in preterm IUGR.
Method
This was a prospective cohort study of growth-restricted fetuses with abnormal umbilical artery (UA) Doppler studies, defined as pulsatility index (PI) >95th percentile for gestational age or absent/reversed end diastolic flow. Fetuses were followed with weekly BPP, and Doppler evaluation of the UA, middle cerebral artery (MCA) and ductus venosus (DV) until the time of delivery. The cerebro-placental Doppler ratio (CPR) was then calculated (MCA PI/UA PI). MCA PI <5th percentile, MCA peak systolic velocity (PSV) >1.5 multiples of the median, DV PI >95th percentile with or without absent/reversed flow, and CPR <1.08 were considered abnormal. Using logistic regression modeling, a weighted scoring index for the prediction of a composite fetal vulnerability index (FVI) which included 5-minute Apgar score <3, cord pH <7.2, seizures, necrotizing enterocolitis, grade 4 intraventricular hemorrhage, periventricular leukomalacia, and neonatal death was developed. A receiver-operating characteristic curve (ROC) was used to identify the best score associated with the FVI.
Results
Of 66 patients meeting inclusion criteria over a 5-year period, 17 (25.8%) had a positive FVI. Abnormal BPP (<8), MCA PI, MCA PSV, DV PI, and CPR were observed in 6%, 27.3%, 13.6%, 56.1% and 33.3% of patients, respectively. From the logistic regression model, a CSTI was developed including a score of 1 for abnormal BPP; 3 for MCA PSV, 1 for DV, 6 for CPR, and 3 for oligohydramnios. The ROC curve identified a score of ≥7 to be the best predictor of FVI with sensitivity of 35.1% and specificity of 91.8% and a positive likelihood ratio of 4.3 (area under ROC 0.73). These test characteristics were better than those for any of the individual component antenatal tests.
Conclusion
Although this novel scoring system performs modestly in predicting adverse outcomes in FGR, it appears to perform better than any individual antenatal test currently available.
Keywords: preterm fetal growth restriction, Doppler evaluation, Biophysical profile, adverse pregnancy outcomes
Fetal growth restriction (FGR) is a significant contributor to perinatal morbidity and mortality.1 Following a diagnosis of FGR, the goal of prenatal care is close surveillance of the pregnancy with the aim of avoiding intra-uterine fetal death and other adverse prenatal and neonatal outcomes.2
The ideal protocol for monitoring these high-risk pregnancies and the optimal trigger for delivery is still controversial.3,4 This problem is compounded by the potential risk of iatrogenic neonatal morbidity and mortality in the preterm period secondary to early delivery.
One major limitation influencing identification of the best antenatal test for timing the delivery of the preterm FGR pregnancy involves the poor predictive ability of the currently available antenatal surveillance modalities. While some have depended on the biophysical profile (BPP) for monitoring these pregnancies, this test has been shown to be limited in the prediction of adverse outcomes in preterm FGR.5 Others have proposed Doppler surveillance of these cases. However, the results of the only randomized trial addressing delivery timing of the preterm fetus with FGR (GRIT study) also demonstrated the limitations of Doppler assessments.6.7 The GRIT study enrolled FGR singletons or twins between 24–36 weeks if the responsible clinician was uncertain about delivery timing and umbilical artery Doppler had been recorded. Randomization was to immediate delivery (within 48 hours allowing for steroid administration), or delay until the physician felt delivery could no longer be safely deferred. The main outcome measures were perinatal mortality and Griffith’s developmental quotient at age 2. Over 500 women were recruited in the study. 6 Deaths prior to discharge were identical in both groups and total cesarean sections for delivery were more frequent when immediate delivery was required. Ninety-eight percent completed 2 year follow up which showed comparable rate of death or disability (16–19%) in both arms.7
In this study, we sought to test the hypothesis that combining features of the biophysical profile (BPP) and multi-vessel Doppler evaluation into a single combined sonographic testing index (CSTI), improves prediction of adverse outcomes in preterm FGR compared with using the individual components of the index alone.
Methods
This was a prospective cohort study of preterm growth-restricted fetuses with abnormal umbilical artery (UA) Doppler studies at Washington University in Saint Louis Medical Center. Women were included in the study if they carried singleton pregnancies between 26 0/7 and 36 6/7 weeks gestation and had had pregnancy dating confirmed by ultrasound prior to 20 weeks gestation. Women were excluded if they were carrying a multiple gestation, had a fetus with either a structural abnormality or an abnormal fetal karyotype. The study was conducted with the approval of the Washington University Human Research Protection Office.
Fetal growth-restriction was defined as an estimated fetal weight < 10th percentile for the gestational age(GA) using the birth weight nomogram by Alexander et al.8 The actual fetal weight estimates were generated using Hadlock’s formula. 9 Fetuses were followed with weekly BPP, and Doppler evaluation of the UA, middle cerebral artery (MCA) and ductus venosus (DV) until the time of delivery. Umbilical artery waveforms were obtained from a free loop of the umbilical cord. We defined abnormal UA Doppler as either a pulsatility index (PI) >95th percentile for gestational age (GA) or absent/reversed end diastolic flow, The MCA waveforms were obtained from the proximal portion of the vessel as it arises from the circle of Willis, with the angle of insonation as close to 0 degree as possible. MCA PI <5th percentile for GA or with a peak systolic velocity (PSV) >1.5 multiples of the median was considered abnormal. The cerebro-placental Doppler ratio (CPR) was then calculated using the formula: MCA PI/UA PI. The DV waveforms were obtained from a transverse view of the fetal abdomen in the same plane as the abdominal circumference. A DV PI >95th percentile with or without absent/reversed flow, and CPR <1.08 were considered abnormal.10,11,12 Doppler waveforms were obtained by trained RDMS sonographers. All diagnoses of FGR and Doppler interpretations were made by qualified perinatologists. The last Doppler or test assessment prior to delivery was used for the analyses.
The primary outcome for this study was the prediction of a fetal vulnerability index (FVI) which is a composite set of variables, including the following: intrauterine-fetal death, a 5-minute Apgar score <3, cord arterial pH <7.2, seizures, necrotizing enterocolitis, grade 3 or 4 intraventricular hemorrhage, periventricular leukomalacia, and neonatal death. Neonatal outcomes were obtained by reviewing delivery and neonatal medical records. Diagnoses of the individual neonatal outcomes were made by the Attending Neonatologist.
In our statistical analysis, descriptive statistics including chi-square or Fishers exact test were used to compare categorical variables as appropriate and student t-test for continuous variables. In developing the CSTI, we first used logistic regression modeling to develop a weighted scoring index for the prediction of the FVI by the individual biophysical tests. The weighting was based on a factor of the coefficient for each variable in the model. The factor with the lowest coefficient was given a score of 0.5 and the others given scores based on multiples of the lowest coefficient: i.e. CSTI = (coefficient of variable from logistic regression model/coefficient of variable with the lowest coefficient) x0.5. MCA PI was not included in the CSTI score as it was already a component of the CPR to avoid co-linearity. The final scores were rounded down to the nearest whole digit. We then used receiver-operating characteristic curve (ROC) to identify the best score associated with the FVI. The results of the CSTI score were not available to providers and not used for clinical management. All statistical analyses were performed using STATA (version 10.0, Stata corp., College Station, TX) and a p-value of <0.05 was considered significant.
Results
Over a 5-year period, 1620 patients with FGR were identified, and 66 of these met the inclusion criteria. The rest were excluded either due to multiple gestation, fetal abnormality, aneuploidy of normal umbilical artery Doppler. The most common reason for exclusion was a normal umbilical artery Doppler. The demographic characteristics of those included in the study are shown in Table 1. The majority of the patients were African American and nulliparous, with a mean gestational age at diagnosis of IUGR of 31 weeks. There were 17 (25.8%) who had at least one positive component of the FVI. These included: 2 (3%) cases of intrauterine-fetal death; 4 (6%) with a 5-minute Apgar score <3; 10(15%) with cord arterial pH <7.2; 0 (0%) seizures; 3(4.5%) with necrotizing enterocolitis; 1(1.5%) grade 3 or 4 intraventricular hemorrhage; 1 (1.5%) periventricular leukomalacia; and 3 (4.5%) neonatal deaths.
Table 1.
Demographics and admission characteristics of study population.
| Characteristics | N=66 | % |
|---|---|---|
|
| ||
| Maternal age (mean years ±SD) | 24.9 (6.3) | - |
|
| ||
| Multiparous | 25 | 37.9 |
|
| ||
| Race | ||
| African American | 39 | 59.1 |
| White | 25 | 37.9 |
| Hispanic | 1 | 1.5 |
| Asian | 1 | 1.5 |
|
| ||
| Smoking | 23 | 34.9 |
|
| ||
| Chronic hypertension | 20 | 30.3 |
|
| ||
| Pregestational diabetes | 4 | 6.1 |
|
| ||
| Mean gestational age at enrollment in weeks (±SD) | 31.1 (3.2) | - |
|
| ||
| Mean gestational age at delivery in weeks (±SD) | 34.1 (3.5) | - |
|
| ||
| Mean birth weight, g (±SD) | 1778.3 (678.7) | - |
|
| ||
| Indications for delivery* | ||
| Preeclampsia | 10 | 15 |
| Abnormal BPP | 4 | 6 |
| Absent or reversed umbilical artery Doppler | 15 | 23 |
| Non-reassuring non-stress test | 5 | 7 |
| IUGR at 37 weeks | 35 | 53 |
Total exceeds 100% as some cases had more than one indication for delivery.
Abnormal BPP (<8), MCA PI, MCA PSV, DV PI, and CPR were observed in 6%, 27.3%, 13.6%, 56.1% and 33.3% of patients, respectively. From the logistic regression model, a CSTI was developed including a score of 3 for MCA PSV, 1for DV, 6 for CPR, and 3 for oligohydramnios (Table 2). BPP was not associated with a positive FVI, and was therefore not included in the final CSTI despite the historical use of this test for monitoring cases with FGR.
Table 2.
Logistic regression model for deriving the combined sonographic scoring index
| Sonographic criterion | Coefficient | 95% CI | Standard error |
|---|---|---|---|
| BPP score | - | - | - |
| Oligohydramnios | 0.92 | −0.40–2.2 | 0.67 |
| MCA PSV | 0.99 | −0.67–2.66 | 0.85 |
| Ductus venosus | 0.15 | −1.15–1.45 | 0.66 |
| CPR <1.08 | 1.86 | 0.37–3.36 | 0.76 |
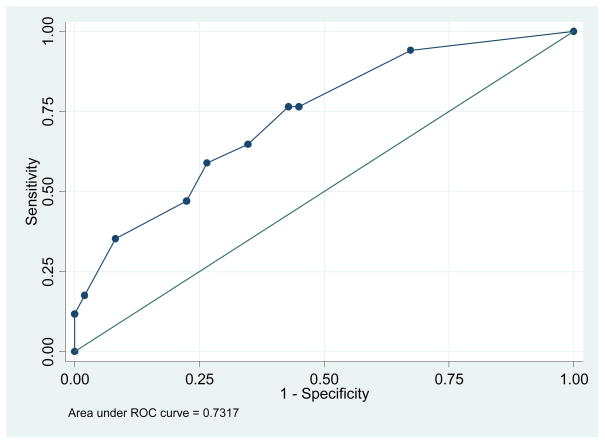
The ROC curve (figure 1) identified a score of ≥7 to be the best predictor of FVI with sensitivity of 35.1% and specificity of 91.8% and a positive likelihood ratio of 4.3 (area under ROC 0.73). These test characteristics for the CSTI were better than those for any of the individual component antenatal tests (Table 3). The BPP score had a similar specificity to the CSTI, but did not predict any pregnancy at risk for a FVI. The CPR and other parameters including the MCA demonstrated acceptable test characteristics.
Figure 1.
ROC curve evaluating the predicting efficiency of the combined sonographic testing index.
Table 3.
Comparing individual antenatal tests to the combined sonographic screening index
| Test | Sensitivity (%) | Specificity (%) | LR+(95% CI) | AUC (95% CI) |
|---|---|---|---|---|
| BPP score | 0 | 91.8 | 1.1(0.6–1.8) | 0.46 (0.42–0.50) |
| MCA PI<5th percentile | 35.3 | 75.5 | 1.4(0.8–2.2) | 0.55 (0.42–0.55) |
| MCA PSV > 1.5 MoM | 23.5 | 89.8 | 2.3 (1.5–3.2) | 0.57 (0.45–0.68) |
| CPR <1.08 | 58.8 | 75.5 | 2.4 (1.6–3.4) | 0.67 (0.54–0.81) |
| Abnormal ductus venosus | 58.8 | 44.9 | 1.1 (0.6–1.7) | 0.52 (0.38–0.66) |
| CSTI >7 | 35.3 | 91.8 | 4.3 (3.3–5.3) | 0.73 (0.59–0.87) |
Discussion
We found the CSTI to be better at identifying pregnancies at risk for a composite of adverse outcomes compared with using BPP or Doppler indices alone. Abnormal BPP score was not associated with an adverse outcome in this cohort. When the Doppler modalities are evaluated individually, the CPR which combines the umbilical artery and middle cerebral artery PI was better at identifying those at risk for adverse outcome.
The sensitivity and specificity of the CSTI was however only modest. The ideal screening test for adverse perinatal outcome in fetuses with preterm growth-restriction should ideally have a high sensitivity and specificity. However given the significance of these adverse outcomes following a preterm FGR delivery, a test with a reasonable specificity but high sensitivity could be adopted clinically. Of the tests evaluated, only the CPR would be a clinically plausible alternative to the CSTI. The search for the best test to trigger delivery of the preterm growth-restricted fetus has been the challenge of perinatal researchers for many years. This study offers the CSTI as a candidate for further evaluation.
Strengths of the current study include the detailed follow-up of cases identified with FGR and abnormal umbilical artery Doppler without providing the clinicians with results of the ductus venosus or middle cerebral artery findings. This allowed us to observe the natural history of cases with abnormalities of these adjuvant Doppler indices without biasing clinicians towards premature intervention. Withholding these data from clinicians was ethically justified in our unit where these additional Doppler evaluations are not currently considered the standard of care for cases with FGR.
Our study is not without limitations. The most important one is the sample size and slow accrual of subjects over a long period. Consequently, we used a composite of adverse outcomes for our primary outcome. The small sample size of our study however suggests that the results must be interpreted with caution as larger studies powered for individual adverse neonatal outcomes may produce different results. The sample size limitation is reflected on the finding that despite the improved point estimate of the area under the ROC curve for the CSTI, the confidence intervals overlaps with many of the individual antenatal tests. Finally, while the CSTI score was not available to managing clinicians, some of the current tests such as BPP, amniotic fluid volume and the umbilical artery Doppler were available to them and were used for clinical decisions regarding delivery as Table 1 illustrates. This however, did not detract from evaluating the ability of such tests to predict adverse outcomes. For example, while four patients were delivered for abnormal BPP scores, none of these had an adverse outcome.
The study illustrates that a multi-centered large study will be ideal to provide a definitive answer to guide clinicians caring for these patients. The ongoing TRUFFLE (Trial of Umbilical and Fetal Flow in Europe) study in Europe illustrates some of the practical and ethical issues of conducting such a prospective trial.12 Interestingly, ductus venosus flow (which is one of the triggers for delivery being studied in that trial) was not found to be significant in identifying those at risk for adverse outcome in the current study. Unfortunately, the TRUFFLE trial will not provide an answer to whether a combination of Doppler and biophysical profile can be used to monitor and time the delivery of preterm FGR as these are not in any arms of the study.
In conclusion, the combined sonographic testing index demonstrates a modest ability to identify cases of FGR at risk for adverse outcome when identified in the preterm period. Further studies are needed to validate these findings prior to adopting the index clinically.
Acknowledgments
The study was supported by a grant from the Barnes Jewish Hospital Foundation.
References
- 1.Baschat AA, Galan HL, Bhide A, Berg C, Kush ML, Oepkes D, Thilaganathan B, Gembruch U, Harman CR. Doppler and biophysical assessment in growth restricted fetuses: distribution of test results. Ultrasound Obstet Gynecol. 2006 Jan;27(1):41–7. doi: 10.1002/uog.2657. [DOI] [PubMed] [Google Scholar]
- 2.Baschat AA, Guclu S, Kush ML, Gembruch U, Weiner CP, Harman CR. Venous Doppler in the prediction of acid-base status of growth-restricted fetuses with elevated placental blood flow resistance. Am J Obstet Gynecol. 2004 Jul;191(1):277–84. doi: 10.1016/j.ajog.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 3.Baschat AA, Odibo AO. Timing of delivery in fetal growth restriction and childhood development: some uncertainties remain. Am J Obstet Gynecol. 2011 Jan;204(1):2–3. doi: 10.1016/j.ajog.2010.10.915. [DOI] [PubMed] [Google Scholar]
- 4.Boers KE, Vijgen SM, Bijlenga D, et al. Induction versus expectant monitoring for intrauterine growth restriction at term: randomised equivalence trial (DIGITAT) BMJ. 2010 Dec 21;341:c7087. doi: 10.1136/bmj.c7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaur S, Picconi JL, Chadha R, Kruger M, Mari G. Biophysical profile in the treatment of intrauterine growth-restricted fetuses who weigh <1000 g. Am J Obstet Gynecol. 2008;199:264, e1–4. doi: 10.1016/j.ajog.2008.06.074. [DOI] [PubMed] [Google Scholar]
- 6.GRIT Study Group. A randomised trial of timed delivery for the compromised preterm fetus: short term outcomes and Bayesian interpretation. BJOG. 2003 Jan;110(1):27–32. doi: 10.1046/j.1471-0528.2003.02014.x. [DOI] [PubMed] [Google Scholar]
- 7.Thornton JG, Hornbuckle J, Vail A, Spiegelhalter DJ, Levene M GRIT study group. Infant wellbeing at 2 years of age in the Growth Restriction Intervention Trial (GRIT): multicentred randomised controlled trial. Lancet. 2004 Aug 7–13;364(9433):513–20. doi: 10.1016/S0140-6736(04)16809-8. [DOI] [PubMed] [Google Scholar]
- 8.Alexander GR, Hime JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–8. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 9.Hadlock FP, Harrist RB, Martinez-Poyer J. In-utero analysis of fetal growth: a sonographic weight standard. Radiology. 1991;181:129–33. doi: 10.1148/radiology.181.1.1887021. [DOI] [PubMed] [Google Scholar]
- 10.Acharya G, Wilsgaard T, Berntsen GK, Maltau JM, Kiserud T. Reference ranges for serial measurements of umbilical artery Doppler indices in the second half of pregnancy. Am J Obstet Gynecol. 2005;192:937–44. doi: 10.1016/j.ajog.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 11.Odibo AO, Riddick C, Pare E, Stamilio DM, Macones GA. Cerebroplacental Doppler ratio and adverse perinatal outcomes in intrauterine growth restriction: evaluating the impact of using gestational age-specific reference values. J Ultrasound Med. 2005 Sep;24(9):1223–8. doi: 10.7863/jum.2005.24.9.1223. [DOI] [PubMed] [Google Scholar]
- 12.Mari Giancarlo, Hanif Farhan. Fetal Doppler: Umbilical Artery, Middle Cerebral Artery, and Venous System. Semin Perinatol. 2008;32:253–7. doi: 10.1053/j.semperi.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Lees C, Baumgartner H. The TRUFFLE study--a collaborative publicly funded project from concept to reality: how to negotiate an ethical, administrative and funding obstacle course in the European Union. Ultrasound Obstet Gynecol. 2005;25:105–7. doi: 10.1002/uog.1836. [DOI] [PubMed] [Google Scholar]