Abstract
Objectives
Gentamicin (GM) is a commonly used aminoglycoside antibiotic that generates free oxygen radicals within the inner ear, which can cause vestibulo-cochlear toxicity and permanent damage to the sensory hair cells and neurons. Piper longum L. (PL) is a well-known spice and traditional medicine in Asia and Pacific islands, which has been reported to exhibit a wide spectrum of activity, including antioxidant activity. In this study, we evaluated the effect of hexane:ethanol (2:8) PL extract (subfraction of PL [SPL] extract) on GM-induced hair cell loss in basal, middle and apical regions in a neonatal cochlea cultures.
Methods
The protective effects of SPL extract were measured by phalloidin staining of cultures from postnatal day 2-3 mice with GM-induced hair cell loss. The anti-apoptosis activity of SPL extract was measured using double labeling by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) and myosin-7a staining. The radical-scavenging activity of SPL extract was assessed using the 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay.
Results
SPL extract at a concentration of 1 µg/mL significantly inhibited GM-induced hair cell loss at basal and middle region of cochlea, while 5 µg/mL was effective against apical region hair cell loss. The protective effect of SPL extract was concentration dependent and hair cells retained their stereocilia in explants treated with SPL extract prior to treatment with 0.3 mM GM. SPL extract decreased GM-induced apoptosis of hair cells as assessed by TUNEL staining. The outer hair and inner hair counts were not decreased in SPL extract treated groups in compare to GM treated explants. Additionally, SPL extract showed concentration dependent radical scavenging activity in a DPPH assay.
Conclusion
An anti-apoptosis effect and potent radical scavenger activity of SPL extract protects from GM-induced hair cell loss at basal, middle and apical regions in neonatal cochlea cultures.
Keywords: Gentamicin, Autotoxicity, Inner hair cell, Outer hair cell, Piper longum, Apoptosis, Antioxidant
INTRODUCTION
Aminoglycoside antibiotics, including gentamicin (GM), display ototoxicity resulting in sensorineural hearing loss and dizziness [1]. Inner ear hair cells, which convert sound signals into electrical impulses, are vulnerable to the damage caused by aminoglycoside antibiotics [2]. Apoptosis is the predominant mode of inner ear hair cell loss and reactive oxygen species (ROS) play an important role in the process of apoptosis [3].
Several agents have shown protective effects on ototoxicity by blocking the formation of ROS or scavenging ROS. These include vitamin E, α-lipoic acid, Ebselen and melatonin [2,4-6]. Various herbal extracts have been investigated as protective agents against aminoglycoside-induced ototoxicity. These include Ginkgo biloba, Gu Sui Bu and Tanshinone, a phenolic acid derivative of Danshen [7-9].
In a previous study, we showed that the ethanol extract of Piper longum L. (PL) had a protective effect on GM-induced hair cell loss in neonatal cochlea cultures [10]. The natural compound is usually a complex mixture and subfractions from the crude extract can show different biologic activities. Thus, we hypothesized that subfractions of ethanol extract of PL may have biologic characteristics different from the total ethanol extract of PL, and may show higher protective effect on GM-induced hair cell loss in neonatal cochlea cultures.
To assess the hypothesis, subfractions of the ethanol extract of PL were generated using various solvents and their anti-oxidative effect and protective effect on GM-induced hair cell loss in neonatal cochlea cultures were evaluated. In particular, we report results from the subfraction generated using hexane:ethanol (H:E) in a volume ratio of 2:8.
MATERIALS AND METHODS
Ethanol extract of PL
Authenticated PL was obtained from the Department of Pharmacy, Dongguk University Ilsan Hospital. Dried fruit powder was extracted twice in 10 volumes of 30% ethanol for 3 hours at 85℃-90℃, and filtered with a 40 µm filter. Ethanol was removed in a vacuum at 60℃ and the yield obtained was 4.7%. The extract was resuspended in phosphate buffered saline (PBS, pH 7.2).
Subfractions of ethanol extract of PL
Subfractions of the ethanol extract of PL were extracted using various combinations of hexane and ethanol for 3 hours at 85℃-90℃, and filtered as described above. Solvent was removed in a vacuum at 60℃. The subfraction generated using H:E in a volume ratio of 2:8 (subfraction of PL [SPL] extract) was assessed for anti-oxidative effect and protective effect on GM-induced hair cell loss in neonatal cochlea cultures.
Cochlea organotypic culture
Cochlea organotypic culture was made as described previously [10]. All protocols were in accordance with the guidelines of the Animal Care Ethics Committee of Dongguk University Ilsan Hospital guidelines. ICR mouse (Koatech, Pyeongtaek, Korea) pups were decapitated on postnatal day 4. The cochlea was carefully dissected as a flat-surface preparation of the organ of corti. A drop of rat tail collagen (type 1, 3.86 mg/mL in 0.02 N acetic acid; BD Biosciences, Franklin Lakes, NJ, USA), 10x basal medium eagle (Sigma-Aldrich, St. Louis, MO, USA), and 2% sodium carbonate at a 9:1:1 ratio was placed in a 35 mm-diameter culture dish (Nunc, Rochester, NY, USA) and allowed to gel. Afterwards, 1 mL of serum-free Dulbecco's modified Eagle's medium (Welgene, Daegu, Korea) supplemented with 1% N-1 supplement (Sigma-Aldrich) and 50 U/mL penicillin G (Sigma-Aldrich) was added. The organ of corti was gently pressed onto the surface of the collagen gel with forceps and held in place by the surface tension of the culture medium. Cochlea cultures were incubated at 37℃ in a humidified atmosphere of 5% CO2 overnight and then exposed to a medium containing GM (0.3 mM) for 24 hours. To evaluate the protective effect, the explants were treated with SPL extract (0, 1, 5, and 10 µg/mL) for 1 hour, prior to GM treatment for 24 hours.
Hair cell counts
After incubation for 24 hours, the cultures were fixed, permeabilized, and stained with Alexa Fluor 350 phalloidin (Invitrogen, Carlsbad, CA, USA). The explants were then mounted on a slide with Gel Mount Biomedia (Fisher Scientific, Waltham, MA, USA), coverslipped, and examined with a DMIL microscope (Leica, Jena, Germany). Each labeled hair cell cilia was counted in the three outer hair cells (OHC) rows and one inner hair cell (IHC) row. Cells were considered to be missing if there was a gap in the normal geometric array and no stereocilia or cuticular plates were apparent. The number of cells was counted over a distance of 100 µm from five randomly selected fields for the basal, middle and apical turns of each explant. The average from five fields was considered as a single sample, and typically 5-11 specimens were evaluated for each condition.
TUNEL and myosin-7a double labeling
The anti-apoptosis effect was assessed with terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL). DNA fragmentation was assessed in situ using a TUNEL staining kit (Trevigen, Gaithersburg, MD, USA) as described by the manufacturer, with minor modifications. Fixed cochlea organotypic cultures were placed in 0.01 M PBS for 10 minutes, and treated with proteinase K solution for at least 15 minutes at room temperature. After washing twice with deionized water for 2 minutes, each cultures was immersed in 1× TdT labeling buffer for 5 minutes. Cultures were incubated in TUNEL mix containing 50× TdT dNTP, 50× cation (Mg2+), 50× TdT enzyme and 1× TdT labeling buffer for 60 minutes at 37℃ in a humidity chamber. The reaction was terminated by washing in 1× TdT stop buffer for 5 minutes. The cultures were washed in 0.01 M PBS-Tween (PBS-T) and treated with Strep-Fluor solution for 30 minutes at room temperature in the dark, before being mounted on glass with coverslips using fluorescence mounting medium. Samples were viewed under a fluorescence microscope using a 495 nm filter. For myosin-7a labeling, the cultures were fixed, permeabilized, and stained with myosin-7a rabbit polyclonal antibody (1:300 dilution; Proteus BioSciences, Ramona, CA, USA) followed by Alexa Fluor 594 donkey anti-rabbit IgG (1:500 dilution; Invitrogen). The explants were mounted on a slide with Gel Mount Biomedia (Fisher Scientific), coverslipped, and examined with a model DP70 fluorescence microscope (Olympus, Tokyo, Japan). Five wells in a 96-well plate were used for each concentration. Results were obtained from three repeated experiments.
2,2-Diphenyl-1-picrylhydrazyl (DPPH) assay
The evaluation of free radical scavenging activity was conducted. The SPL extract stock was diluted in 99% ethanol to make 1, 5, 10 µg/mL concentrations respectively. DPPH (100 mM; Sigma Aldrich) solution was prepared in 95% ethanol. The SPL extract was diluted twice in 50% ethanol and DPPH diluted 100-fold in 95% ethanol (DPPH solution). A 0.5 mL volume of sample was mixed with 3.0 mL (1 mM) DPPH and allowed to stand at room temperature for 30 minutes under light protection. The absorbance was measured at 540 nm with spectrophotometer (Molecular Devices, Sunnyvale, CA, USA). The difference in absorbance between the test and the control was calculated and expressed as percentage (%). The capability to scavenge the DPPH radical was calculated by using the following equation:
Statistical analysis
The results data are expressed as mean±SD. A student t-test was used to determine the statistically significant differences between the groups. A P<0.05 was classified as significant.
RESULTS
Protective effect of SPL extract on GM-induced hair cell loss
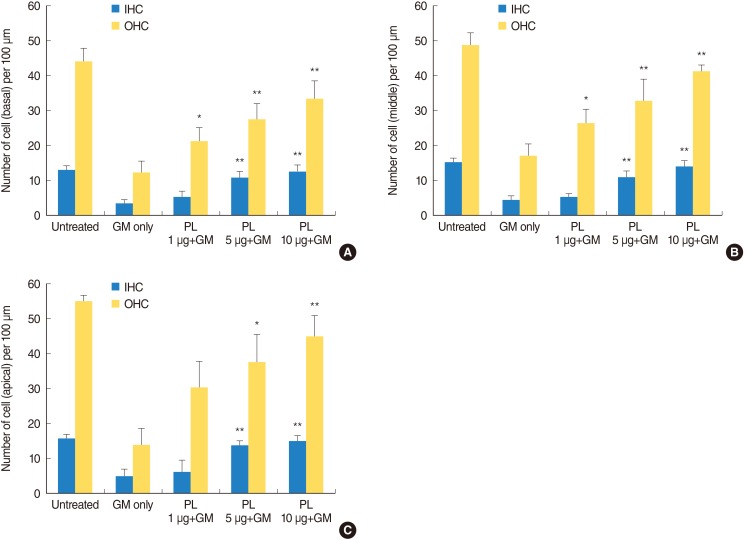
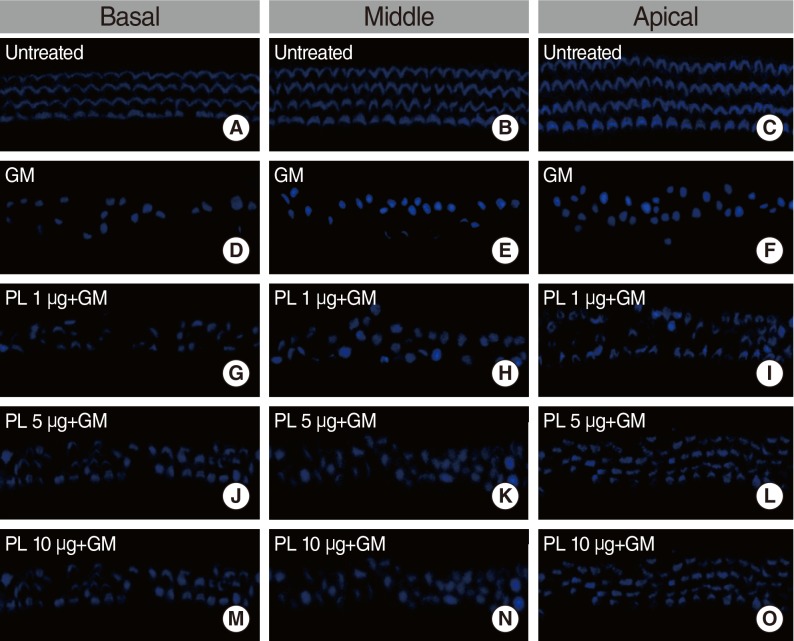
Cochlear cultures from postnatal mice were incubated in medium containing SPL extract at concentrations of 1, 5, and 10 µg/mL for 1 hour, followed by GM (0.3 mM) treatment. Viable phalloidin-labeled hair cells were counted after 24 hours. In GM treated groups, the OHC and IHC counts were significantly decreased. No such decrease in the OHC and IHC were detected in explants treated with SPL extract. The SPL extract at concentrations of 1 µg/mL and 5 µg/mL significantly (P<0.01) protected OHC and IHC, respectively, at the basal region of cochlea in a dose dependent manner (Fig. 1A). Similarly, the SPL extract at concentrations of 1 µg/mL and 5 µg/mL demonstrated a significant (P<0.01) protective effect against GM-induced OHC and IHC loss at the middle region of cochlea (Fig. 1B). SPL extract at concentration of 5 µg/mL was significantly (P<0.01) protective for OHC and IHC of the apical part of cochleae (Fig. 1C). Compared with untreated controls (Fig. 2A-C), GM severely distorted the anatomy of the organ of corti in the GM-treated group. In this group, hair cells were deformed and deranged, and gaps were visible (Fig. 2D-F). The SPL extract was not toxic to the cochlea and reduced GM-induced cytotoxicity (Fig. 2G-O); the alignment of cells in the organ of corti was somewhat irregular, but hair cells retained their shape and stereocilia in SPL extract treated group (Fig. 2G-O). These results indicated that the SPL extract was significantly protective against GM-induced hair cell loss, and this protective effect was more significant on the OHC than IHC. Among the basal, middle and apical regions of the cochleae, the protective effect of SPL extract was more significant on basal and middle hair cells than apical hair cells.
Fig. 1.
Phalloidin labeled outer hair cell and inner hair cell count. (A-C) show hair cell counts per 100 µm length of basal, middle and apical part of cochlea, respectively. Bundles labeled with phalloidin strain were counted under a fluorescence microscope and are presented as the mean±SD. The hair cell count of a single sample was the average of five fields, and 5-11 specimens were assessed for each condition. The results were significant (*P<0.01, **P<0.005) as compared with the gentamicin-alone group. IHC, inner hair cell; OHC, outer hair cells; GM, gentamicin; PL, Piper longum L.
Fig. 2.
Microscopic images of phalloidin labeled inner hair cell and outer hair cell. All examples are from comparable basal, middle and apical regions of explants. (A-C) depict basal, middle and apical region of explants of untreated group. (D-F) depict basal, middle and apical region of gentamicin (GM; 0.3 mM) treated group. (G-I) depict basal, middle and apical region of 1 µg subfraction of SPL extract and GM treated group. (J-L) depict basal, middle and apical region of 5 µg SPL extract and GM treated group. (M-O) depict basal, middle and apical region of 10 µg SPL extract and GM treated group. GM, gentamicin; PL, Piper longum L.; SPL, subfraction of PL.
Anti-apoptotic activity of SPL extract
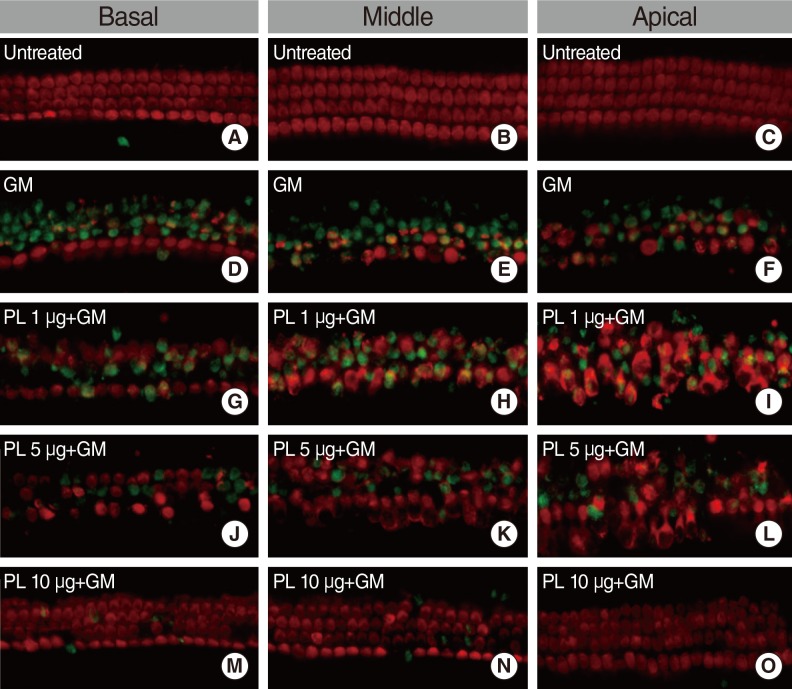
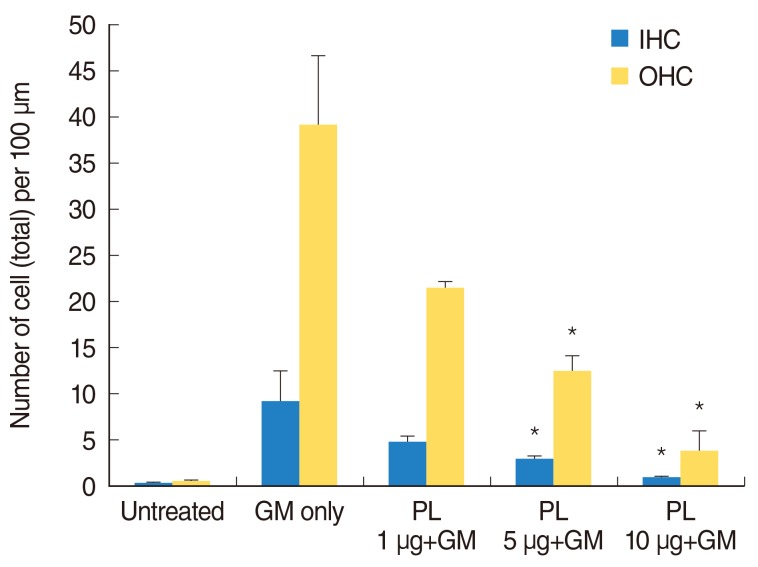
Apoptosis was evaluated by TUNEL assay, which stained DNA fragments in the apoptotic cells and the hair cell loss was detected by myosin-7a labeling. In the untreated group, apoptotic cells were absent and no decrease of OHC and IHC were detected by TUNEL and myosin-7a double labeling (Fig. 3A-C). TUNEL-positive (apoptotic) cells were found in the cochlear culture after 24 hours of 0.3 mM GM treatment (Fig. 3D-F). Myosin-7a labeling detected significant OHC and IHC loss in the basal, middle and apical regions of the cochlea. Pretreatment of the explants with SPL extract (1 µg/mL) significantly reduced the number of TUNEL-positive cells (green fluorescence) and increased hair cell count (red fluorescence) (Fig. 3G-I). The SPL extract at concentrations of 1-10 µg/mL significantly prevented GM-induced apoptotic cell death of both IHCs and OHCs. TUNEL-positive cells were significantly decreased in the explants exposed to 1-10 µg/mL of SPL extract (Fig. 3G-O). The TUNEL labeled OHC and IHC counts were significantly increased in explants treated with 0.3 mM GM. When the explants were treated with the SPL extract prior to GM treatment, the OHC and IHC counts decreased. The anti-apoptosis effect of SPL extract was dose dependent (Fig. 4). At concentration of 5 µg/mL and above, the SPL extract significantly (P<0.05) protected OHC and IHC against GM induced apoptosis and hair cell loss. These results demonstrated the protection conferred by SPL extract on OHC and IHC against GM-induced hair cell loss, and the significant concentration dependent anti-apoptosis activity of the extract.
Fig. 3.
Microscopic images of anti-myosin-7a (red) and TUNEL (green) double-labeled P4 organ of corti explants. All examples are from comparable basal, middle and apical regions of explants. (A-C) depict control group explants. (D-F) show explants treated with gentamicin (0.3 mM). (G-I) show explants treated with 1 µg/mL subfraction of SPL extract and GM. (J-L) show explants treated with 5 µg/mL SPL extract and GM. (M-O) show explants treated with 10 µg/mL SPL extract and GM. GM, gentamicin; PL, Piper longum L.; SPL, subfraction of PL.
Fig. 4.
TUNEL-labeled outer hair cell (OHC) and inner hair cell (IHC) count. The black bar and grey are representing total OHC and IHC counts per 100 µm length of cochlear respectively. TUNEL-labeled bundles were counted under a fluorescence microscope and are presented as the mean±SD of OHCs and IHCs hair cells per 100 µm length of the cochlea. The hair cell count of a single sample was the average of five fields, and 5-11 specimens were assessed for each condition. The results were significant (*P<0.05) as compared with the control group. GM, gentamicin; PL, Piper longum L.
Radical-scavenging activity of SPL extract
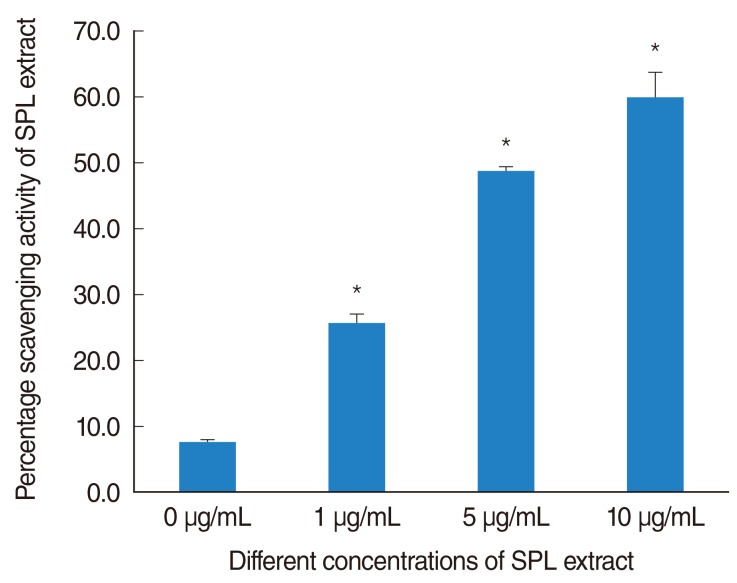
The radical scavenging activity of SPL extract was detected by DPPH assay. A concentration dependent increase in scavenging activity of SPL extract was evident. The percentage scavenging activity of SPL extract was significantly (P<0.05) increased at concentrations ≥1 µg/mL compared with control (Fig. 5). The scavenging activity of 1, 5, and 10 µg/mL of SPL extract was 25.65%, 48.59%, and 60% respectively.
Fig. 5.
Radical scavenging activity of subfraction of Piper longum L. (SPL) extract. The percentage increases in scavenging activity were plotted against the concentration of SPL extract. The results were significant at 1 µg/mL (*P<0.0001). Error bar representing the SD of quadruplicate samples.
DISCUSSION
This study investigated the protective effects of subfraction of ethanol extract of PL on GM-induced hair cell loss in neonatal mouse cochleae cultures. A subfraction of the ethanol extract of PL displayed an anti-oxidative effect in a dose-dependent manner, and protected against GM-induced hair cells loss in the cochleae due to anti-apoptotic activity. Moreover, compared with our previous study [10], hair cell death was protected at a lower concentration (1 µg/mL). The subfraction of ethanol extract of PL possessed more potent anti-oxidative and anti-apoptotic activities than the ethanol extract of PL.
GM is a widely used aminoglycoside antibiotic. However, the clinical utility of aminoglycosides like GM is limited by the potential for ototoxicity and nephrotoxicity [11]. GM can induce hearing loss and balance disturbance due to the destruction of inner ear hair cells [12]. Apoptosis is known as the predominant mode of hair cell loss in ototoxicity and ROS play an important role as a promoter of apoptosis [3]. Aminoglycosides such as GM can form complexes with iron [1] that then react with unsaturated fatty acids to form superoxide (O2) radicals and lipid peroxides [13]. Typically, O2 is converted to hydrogen peroxide by superoxide dismutase and is detoxified into water and oxygen by catalase. However, in highly oxidative conditions, endogenous antioxidant pathways can become overwhelmed and free oxygen radicals can become abundant [14,15]. Aminoglycosides, such as GM, can activate inducible nitric oxide synthase in inner ear tissues, triggering an increase in nitric oxide [6]. Under these conditions, O2 free radical can react with available nitric oxide to form the destructive peroxynitrite radical or it can directly initiate hair cell death.
Hair cell protection is an important target for preventing GM-induced ototoxicity. The common signaling pathways involved in GM-induced ototoxicity are ROS production and apoptosis. Thus, many drugs have been evaluated for hair cell protection from GM-induced ototoxicity by virtue of their prevention of the overproduction of ROS and decreasing apoptosis [10,16-19]. These compounds include iron chelators such desferoxamine and dihydroxybenzoate [20,21] as well as antioxidants such as lipoic acid, a-tocopherol, ebselen, D-methionine, salicylates, and traditional medicines [22]. PL is a well-known traditional medicine in Asia and the Pacific Islands.
PL exhibits a wide spectrum of biologic activities that include anti-amoebic, anti-giardial, immunostimulatory, anti-ulcer, anti-oxidative and anti-inflammatory activities [23]. The major chemical constituents of the PL are volatile oil, resin and alkaloids viz. piperine, piperlongumine and piperlonguminine [24]. Piperine an alkaloid is the major component of Piper longum that possess antioxidant potential against free radical-induced oxidative damage [25,26].
Recently, we reported that the ethanol extract of PL protects against GM-induced hair cell loss [10]. In this previous study, the ethanol extract of PL at concentration of 5 mg/mL was effective against GM-induced OHC and IHC loss of basal and apical region. Here, our results demonstrate that the SPL extract when applied in concentrations as low as 1 µg/mL significantly protects against GM-induced ototoxicity. Moreover, compared with the previously reported extract, enhanced ant-oxidative activity of SPL extract was evident. The main reason for the more potent activity is likely that PL contains polyphenols, whose quantity and quality enhanced in sub-fraction extracted with hexane and ethanol. Furthermore, PL extracted with different solvents showed different biological activity [27-30]. This implies that by optimizing the extraction process, the biologic activity of the natural compound could be enhanced.
In conclusions, SPL extract possessed potent anti-apoptosis effect and potent radical scavenger activity that protects GM-induced hair cells in the basal, middle and apical regions in neonatal cochlea culture.
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (NSSC) (No. 2012M2A2A6035679).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Priuska EM, Schacht J. Formation of free radicals by gentamicin and iron and evidence for an iron/gentamicin complex. Biochem Pharmacol. 1995 Nov;50(11):1749–1752. doi: 10.1016/0006-2952(95)02160-4. [DOI] [PubMed] [Google Scholar]
- 2.Kim JB, Jung JY, Ahn JC, Rhee CK, Hwang HJ. Antioxidant and anti-apoptotic effect of melatonin on the vestibular hair cells of rat utricles. Clin Exp Otorhinolaryngol. 2009 Mar;2(1):6–12. doi: 10.3342/ceo.2009.2.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forge A, Li L. Apoptotic death of hair cells in mammalian vestibular sensory epithelia. Hear Res. 2000 Jan;139(1-2):97–115. doi: 10.1016/s0378-5955(99)00177-x. [DOI] [PubMed] [Google Scholar]
- 4.Fetoni AR, Sergi B, Scarano E, Paludetti G, Ferraresi A, Troiani D. Protective effects of alpha-tocopherol against gentamicin-induced Oto-vestibulo toxicity: an experimental study. Acta Otolaryngol. 2003 Jan;123(2):192–197. doi: 10.1080/00016480310001484. [DOI] [PubMed] [Google Scholar]
- 5.Conlon BJ, Aran JM, Erre JP, Smith DW. Attenuation of aminoglycoside-induced cochlear damage with the metabolic antioxidant alpha-lipoic acid. Hear Res. 1999 Feb;128(1-2):40–44. doi: 10.1016/s0378-5955(98)00195-6. [DOI] [PubMed] [Google Scholar]
- 6.Takumida M, Popa R, Anniko M. Free radicals in the guinea pig inner ear following gentamicin exposure. ORL J Otorhinolaryngol Relat Spec. 1999 Mar-Apr;61(2):63–70. doi: 10.1159/000027643. [DOI] [PubMed] [Google Scholar]
- 7.Wang AM, Sha SH, Lesniak W, Schacht J. Tanshinone (Salviae miltiorrhizae extract) preparations attenuate aminoglycoside-induced free radical formation in vitro and ototoxicity in vivo. Antimicrob Agents Chemother. 2003 Jun;47(6):1836–1841. doi: 10.1128/AAC.47.6.1836-1841.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jung HW, Chang SO, Kim CS, Rhee CS, Lim DH. Effects of Ginkgo biloba extract on the cochlear damage induced by local gentamicin installation in guinea pigs. J Korean Med Sci. 1998 Oct;13(5):525–528. doi: 10.3346/jkms.1998.13.5.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long M, Smouha EE, Qiu D, Li F, Johnson F, Luft B. Flavanoid of Drynaria fortunei protects against gentamicin ototoxicity. Phytother Res. 2004 Aug;18(8):609–614. doi: 10.1002/ptr.1505. [DOI] [PubMed] [Google Scholar]
- 10.Du XF, Song JJ, Hong S, Kim J. Ethanol extract of Piper longum L. attenuates gentamicin-induced hair cell loss in neonatal cochlea cultures. Pharmazie. 2012 Jun;67(6):559–563. [PubMed] [Google Scholar]
- 11.Forge A, Schacht J. Aminoglycoside antibiotics. Audiol Neurootol. 2000 Jan-Feb;5(1):3–22. doi: 10.1159/000013861. [DOI] [PubMed] [Google Scholar]
- 12.Theopold HM. Comparative surface studies of ototoxic effects of various aminoglycoside antibiotics on the organ of Corti in the guinea pig: a scanning electron microscopic study. Acta Otolaryngol. 1977 Jul-Aug;84(1-2):57–64. doi: 10.3109/00016487709123942. [DOI] [PubMed] [Google Scholar]
- 13.Sha SH, Schacht J. Salicylate attenuates gentamicin-induced ototoxicity. Lab Invest. 1999 Jul;79(7):807–813. [PubMed] [Google Scholar]
- 14.Schacht J. Antioxidant therapy attenuates aminoglycoside-induced hearing loss. Ann N Y Acad Sci. 1999 Nov;884:125–130. [PubMed] [Google Scholar]
- 15.Sha SH, Taylor R, Forge A, Schacht J. Differential vulnerability of basal and apical hair cells is based on intrinsic susceptibility to free radicals. Hear Res. 2001 May;155(1-2):1–8. doi: 10.1016/s0378-5955(01)00224-6. [DOI] [PubMed] [Google Scholar]
- 16.Choung YH, Kim SW, Tian C, Min JY, Lee HK, Park SN, et al. Korean red ginseng prevents gentamicin-induced hearing loss in rats. Laryngoscope. 2011 Jun;121(6):1294–1302. doi: 10.1002/lary.21756. [DOI] [PubMed] [Google Scholar]
- 17.Jeong HJ, Choi Y, Kim MH, Kang IC, Lee JH, Park C, et al. Rosmarinic acid, active component of Dansam-Eum attenuates ototoxicity of cochlear hair cells through blockage of caspase-1 activity. PLoS One. 2011 Apr;6(4):e18815. doi: 10.1371/journal.pone.0018815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang J, Jung HH, Yang JY, Choi J, Im GJ, Chae SW. Protective role of antidiabetic drug metformin against gentamicin induced apoptosis in auditory cell line. Hear Res. 2011 Dec;282(1-2):92–96. doi: 10.1016/j.heares.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Park MK, Lee BD, Chae SW, Chi J, Kwon SK, Song JJ. Protective effect of NecroX, a novel necroptosis inhibitor, on gentamicin-induced ototoxicity. Int J Pediatr Otorhinolaryngol. 2012 Sep;76(9):1265–1269. doi: 10.1016/j.ijporl.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 20.Song BB, Schacht J. Variable efficacy of radical scavengers and iron chelators to attenuate gentamicin ototoxicity in guinea pig in vivo. Hear Res. 1996 May;94(1-2):87–93. doi: 10.1016/0378-5955(96)00003-2. [DOI] [PubMed] [Google Scholar]
- 21.Song BB, Sha SH, Schacht J. Iron chelators protect from aminoglycoside-induced cochleo- and vestibulo-toxicity. Free Radic Biol Med. 1998 Jul;25(2):189–195. doi: 10.1016/s0891-5849(98)00037-9. [DOI] [PubMed] [Google Scholar]
- 22.Xie J, Talaska AE, Schacht J. New developments in aminoglycoside therapy and ototoxicity. Hear Res. 2011 Nov;281(1-2):28–37. doi: 10.1016/j.heares.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar S, Kamboj J, Suman, Sharma S. Overview for various aspects of the health benefits of Piper longum linn: fruit. J Acupunct Meridian Stud. 2011 Jun;4(2):134–140. doi: 10.1016/S2005-2901(11)60020-4. [DOI] [PubMed] [Google Scholar]
- 24.Shankaracharya NB, Rao LJ, Naik JP, Nagalakshmi S. Characterization of chemical constituents of Indian Long Pepper (Piper-longum L) J Food Sci Tech. 1997;34(1):73–75. [Google Scholar]
- 25.Pathak N, Khandelwal S. Modulation of cadmium induced alterations in murine thymocytes by piperine: oxidative stress, apoptosis, phenotyping and blastogenesis. Biochem Pharmacol. 2006 Aug;72(4):486–497. doi: 10.1016/j.bcp.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Pradeep CR, Kuttan G. Effect of piperine on the inhibition of nitric oxide (NO) and TNF-alpha production. Immunopharmacol Immunotoxicol. 2003 Aug;25(3):337–346. doi: 10.1081/iph-120024502. [DOI] [PubMed] [Google Scholar]
- 27.Jagdale SC, Kuchekar BS, Chabukswar AR, Lokhande PD, Raut CG. Anti-oxidant activity of Piper longum Linn. Int J Biol Chem. 2009;3(3):119–125. [Google Scholar]
- 28.Joy B, Sandhya CP, Remitha KR. Comparison and bioevaluation of Piper longum fruit extracts. J Chem Pharm Res. 2010;2(4):696–706. [Google Scholar]
- 29.Lokhande PD, Gawai KR, Kodam KM, Kuchekar BS, Chabukswar AR, Jagdale SC. Antibacterial activity of extracts of Piper longum. J Pharm Toxicol. 2007;2(6):574–579. [Google Scholar]
- 30.Sunila ES, Kuttan G. Immunomodulatory and antitumor activity of Piper longum Linn. and piperine. J Ethnopharmacol. 2004 Feb;90(2-3):339–346. doi: 10.1016/j.jep.2003.10.016. [DOI] [PubMed] [Google Scholar]