Figure 1.
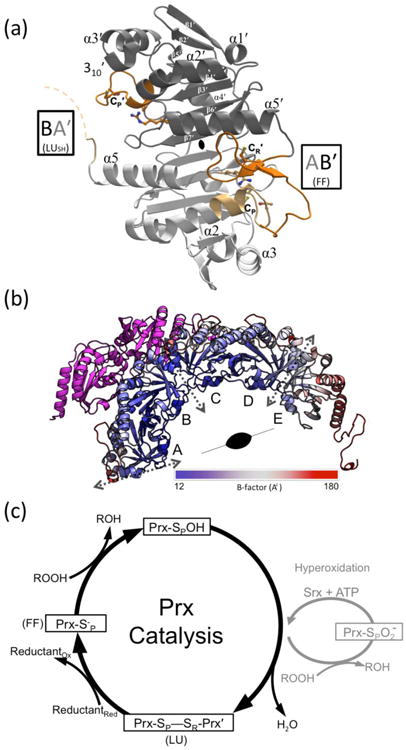
Structure and catalysis of StAhpC. (a) A “B-type” dimer unit of StAhpC with the key catalytic residues depicted as sticks, and select Prx fold core elements of seven β-strands and five helices22 labeled. Chains A (light grey/light orange) and B (dark grey/dark orange) from the WTDTT structure illustrate the FF AB′ active site and the LUSH BA′ active site (seen at ∼50% occupancy). The active site loop (residues 40-50) and C-terminal (residues 161-186) regions are highlighted (orange tones), as is the disordered BA′ C-terminal region (dashed curve). (b) The half-decamer observed in the StAhpC WTDTT asymmetric unit is colored according to mobility from low (blue) to high (red) as indicated. Decamer-building interfaces between B-type dimers (dotted grey two-headed arrows) and the crystallographic two-fold axis that generates the decamer are indicated. Also shown is the symmetry mate hindering folding of the chain A C-terminus (magenta). (c) The Prx catalytic cycle (black) and the hyperoxidation regulatory shunt active in some eukaryotes (grey) are shown. FF and LU conformations involved in catalysis are indicated.
