Figure 4.
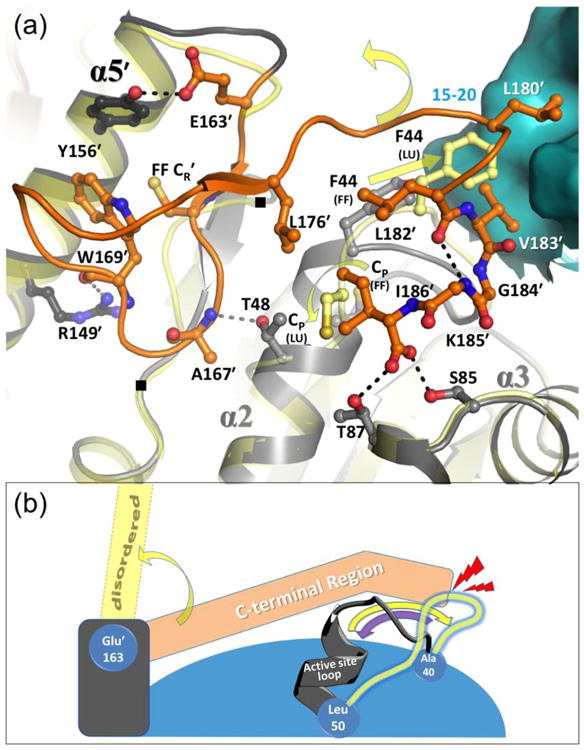
Packing interactions and coordination of unfolding of the FF C-terminal and active site loop regions. (a) The FF active site is shown (active site loop in grey; C-terminal region in dark orange; and the neighboring surface across the decamer-building interface in cyan) along with select interacting residues (labeled and shown as sticks) and stabilizing hydrogen bonds (dashed lines). Positions of residues 137 and 141 are also noted with (■). To provide context, the LUSH conformation (transparent yellow) is shown and yellow arrows indicate movements of the CP residue and Phe44. The C-terminal region has minimal regular secondary structure (residues Val164′–Cys165′ forms a short β-strand and Leu180′-Gly184′ is a short 310-helix), and it interacts with helix α5′ of the same chain (two H-bonds shown), across the decamer-building interface, and with the FF active site loop. The C-terminal residue Ile186′ side chain packs with Pro47, and C-terminal α-carboxylate H-bonds with the side chains of Ser85 and Thr87. (b) Cartoon scheme emphasizing that C-terminal region unfolding destabilizes but does not disrupt the active site loop, whereas active site loop unfolding does disrupt the folding of the C-terminal region. As in panel (a), FF conformations of the two regions are shown in grey and orange, yellow arrows indicate transitions to LU, and LU positions are depicted in transparent yellow. Shown also are approximate hinge points at Ala40, Leu50, and Glu163′ (blue cogs), and the collision of the LU active site loop with the FF C-terminal region (red lightning). The purple arrow emphasizes that the active site loop is not blocked from folding. The decamer-building interactions are not shown in this scheme.
