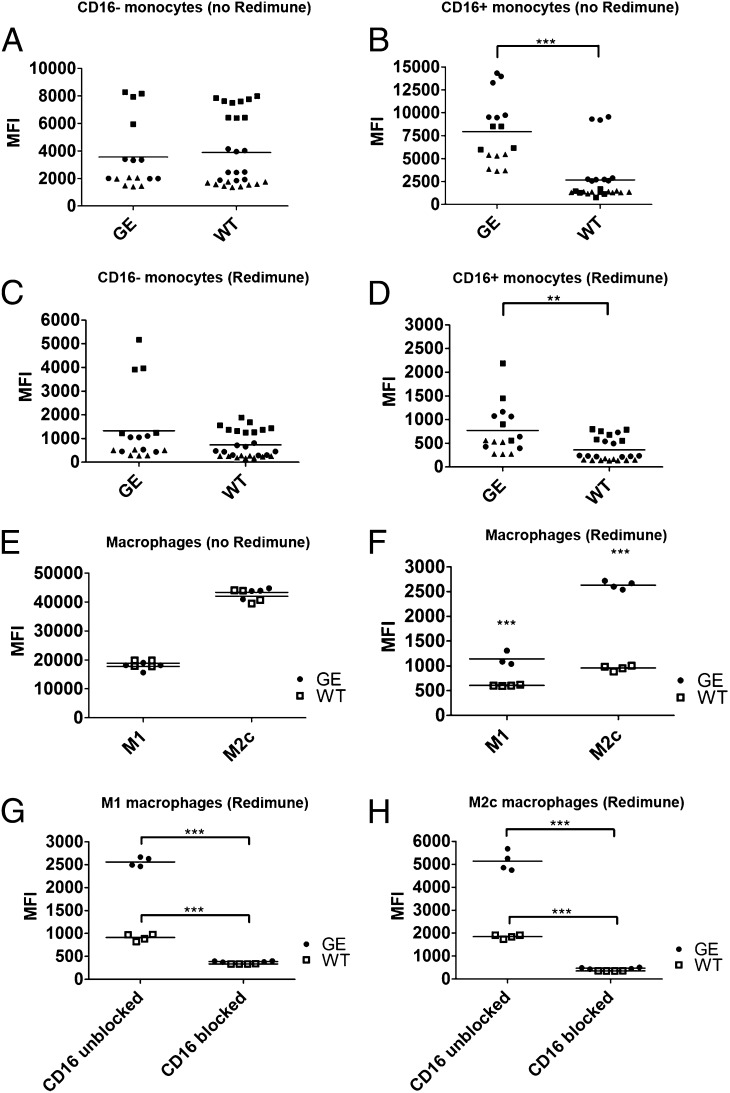
FIGURE 2.
Binding of therapeutic Abs to human monocytes and M1 and M2c macrophages. The binding of AlexaFluor647 directly labeled WT and GE Abs was assessed using classical (CD16−) monocytes (A, C), nonclassical intermediate (CD16+) monocytes (B, D) and M1 and M2c macrophages (E, F). The binding of each Ab was assessed in absence (A, B, E) or presence (C, D, F) of competing endogenous human IgGs (10 mg/ml Redimune). Each symbol in (A)–(D) corresponds to median fluorescence intensity of single data points derived from three independent experiments (donors). Donors are identified by the following symbols: donor 1 = black circles; donor 2 = black squares; donor 3 = black triangles. Two GE Abs were used (GA101 and GA201) as duplicates or triplicates, therefore resulting in four to six symbols per donor per condition. Three WT Abs were used as duplicates or triplicates (GA101 WT, GA201 WT, and ofatumumab), resulting in six to nine symbols per donor per condition. (G and H) Binding of directly labeled WT and GE Abs in the presence of saturating concentrations of blocking CD16 Ab and 10 mg/ml Redimune to M1 (G) and M2c (H) macrophages. Data in (E)–(H) correspond to median fluorescence intensity of duplicates derived from one representative experiment out of a total of three independent ones using GA101 GE, GA201 GE, GA101 WT, and ofatumumab. Two of these experiments included the blocking anti-CD16 Ab. Statistical analysis, unpaired t test: *p < 0.05, **p < 0.01, ***p < 0.001.