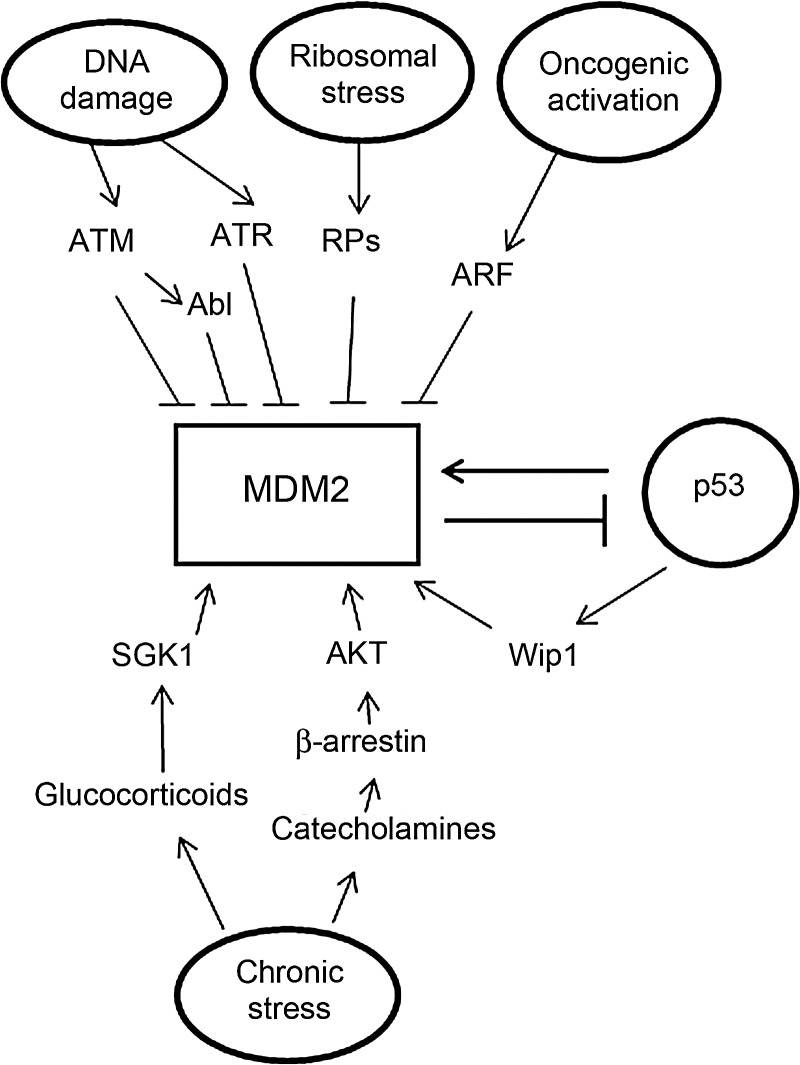
Figure 2.
The regulation of MDM2 protein levels and activity MDM2 protein levels and activity are highly regulated by many stress signals and factors. These stress signals regulate the ability of MDM2 to bind to p53, MDM2 E3 ubiquitin ligase activity, cellular localization of MDM2, and the stability of MDM2 protein. Genotoxic stress signals, such as IR and UV, negatively regulate MDM2 protein levels and activity mainly through post-translational modification (phosphorylation) of MDM2. Oncogenic activation negatively regulates MDM2 E3 ligase activity, and promotes MDM2 localization in nucleoli through ARF. Ribosomal stress signals negatively regulate MDM2 E3 ubiquitin ligase activity through interaction of RPs with MDM2. Some oncogenes, including AKT and Wip1, directly increase MDM2 activity. Chronic psychological stress signals increase the levels of neurohormones, including glucocorticoids and catecholamines, which can increase MDM2 activity through distinct pathways.