Fig. 1.
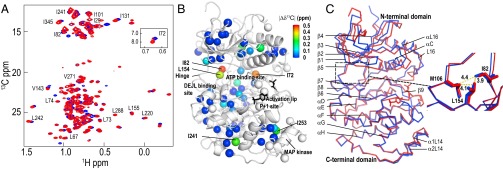
ERK2 phosphorylation induces chemical shift changes at the hinge region. (A) Two-dimensional (13C,1H) HMQC spectra of [methyl-13C]ILV-labeled 0P-ERK2 (blue) and 2P-ERK2 (red) recorded at 800 MHz and 25 °C. (B) X-ray structure of 0P-ERK2 (Protein Data Bank ID code 1ERK) showing spheres representing positions of ILV methyls, with 13C chemical shift differences (|Δδ13C|) between 0P-ERK2 and 2P-ERK2 indicated by the color scale (also see Fig. S2). Unassigned ILV methyls are represented by white spheres. DEJL is the docking site motif for ERK, JNK, and LXL. (C) Structural alignment of 0P-ERK2 (blue) and 2P-ERK2 (red). The expansion shows that the side chains of L154 and I82, which have two of the largest |Δδ13C| (B), form hydrophobic interactions with each other and with M106 at the hinge. Structure images were prepared using PyMOL (www.pymol.org).
