Significance
Glioblastoma multiforme (GBM) is a deadly brain tumor. More than 50% of patients who suffer from GBM die within 15 mo even received all possible medical treatment. In this study we report that the glycolipid stage-specific embryonic antigen-4 (SSEA-4) is highly expressed on the surface of both GBM cells and GBM specimens. We further demonstrate that the growth of GBM tumor is inhibited when anti–SSEA-4 antibody is administered to experimental mice, suggesting a research proof of concept for the treatment GBM and other SSEA-4+ cancers.
Keywords: glycosphingolipids, Globo H, SSEA-3, gangliosides, targeted therapy
Abstract
Glioblastoma multiforme (GBM), the grade IV astrocytoma, is the most common and aggressive brain tumor in adults. Despite advances in medical management, the survival rate of GBM patients remains poor, suggesting that identification of GBM-specific targets for therapeutic development is urgently needed. Analysis of several glycan antigens on GBM cell lines revealed that eight of 11 GBM cell lines are positive for stage-specific embryonic antigen-4 (SSEA-4), and immunohistochemical staining confirmed that 38/55 (69%) of human GBM specimens, but not normal brain tissue, were SSEA-4+ and correlated with high-grade astrocytoma. In addition, an SSEA-4–specific mAb was found to induce complement-dependent cytotoxicity against SSEA-4hi GBM cell lines in vitro and suppressed GBM tumor growth in mice. Because SSEA-4 is expressed on GBM and many other types of cancers, but not on normal cells, it could be a target for development of therapeutic antibodies and vaccines.
Glioblastoma multiforme (GBM), accounting for 60–70% of malignant gliomas, is the most aggressive form of glioma and the most common primary brain tumor in adults (1). Despite treatment, including surgery, and chemo- or radiotherapy, the prognosis for GBM patients is poor, with a median survival rate of 14–15 mo (2). GBM is notoriously resistant to most anticancer drugs and is extremely infiltrative, hampering complete surgical resection; therefore most patients develop tumor recurrence or progression even after multiple therapies. Because of the high mortality, new therapeutic approaches, such as immunotherapy and gene therapy, have been proposed for the treatment of GBM (3).
Altered glycosylation is a feature of cancer cells, and several glycan structures are well-known tumor markers (4, 5). These aberrant changes include the overall increase in the branching of N-linked glycans (6) and sialic acid content (7) and the overexpression of certain glycan epitopes, such as sialyl Lewis x (sLex), sialyl Tn (sTn), Lewis y (Ley), fucosyl Gb5 (Globo H), and polysialic acid (8–10). Many tumors also exhibit increased expression of certain glycolipids, especially the gangliosides, glycosphingolipids (GSLs) with sialic acid(s) attached to the glycan chain. Gangliosides normally are observed in neural systems and are elevated in tumors, particularly the complex gangliosides associated with malignancy (11).
It has been reported that human glioma biopsies show elevation of monosialylated GM3 and GM2 and their disialylated derivatives GD3 and GD2 (12–14). The increase of GD3 was most significant, but the correlation between GD3 and malignancy remains obscure (15, 16). In addition, the lacto-series gangliosides 3′-isoLM1 and 3′,6′-isoLD1 are reported to be major gangliosides in human gliomas (16–18). Because some of these glioma-associated gangliosides are rarely expressed or even are absent in normal tissues (19), they are suitable for targeted therapy (20). Hence, discovering novel glioma-associated GSLs would provide new targets for development of new therapies against gliomas.
The GSLs of globo-series feature a Galα1–4Gal linkage to lactosylceramides, and this linkage is catalyzed by the lactosylceramide 4-alpha-galactosyltransferase, encoded by the A4GALT gene. Although globotriosylceramide (Gb3Cer) and globoside (Gb4Cer) constitute the basis of the P-blood group system (21), galactosyl globoside (Gb5Cer) and sialyl galactosyl globoside (sialyl Gb5Cer, SGG, MSGG), also known as “stage-specific embryonic antigen-3” (SSEA-3) and “stage-specific embryonic antigen-4” (SSEA-4) (22), respectively, are cell-surface markers widely used to define human embryonic stem cells (hESCs). Globo-series GSLs also have been observed in tumors: Globo H is overexpressed in many epithelial cancers [e.g., ovarian, gastric, prostate, lung, breast, and pancreatic cancers (23)]; SSEA-3, SSEA-4, and Globo H are expressed not only on breast cancer cells but also on breast cancer stem cells (24, 25). Moreover, high-level expression of SSEA-4 and disialosyl galactosyl globoside (disialosyl Gb5Cer) is observed in renal cell carcinoma (26), but whether globo-series GSLs are expressed in GBM is not known.
In the present study, we examined the expression levels of globo-series GSLs and several tumor-associated glycans in GBM cell lines by flow cytometry. The result showed that SSEA-4, a ganglioside rarely found in normal brain tissues, was highly expressed on GBM cells and GBM specimens, as confirmed by high-performance TLC (HPTLC) immunostaining and MS. We found that anti–SSEA-4 mAb (MC813-70) could induce complement-dependent cytotoxicity in vitro and inhibit the growth of GBM in nude mice. SSEA-4 is displayed on many other types of cancers and therefore can be a target for the development of therapeutic antibodies and vaccines against SSEA-4+ cancers.
Results
Flow Cytometric Analysis of Glycan Epitopes on GBM Cell Lines.
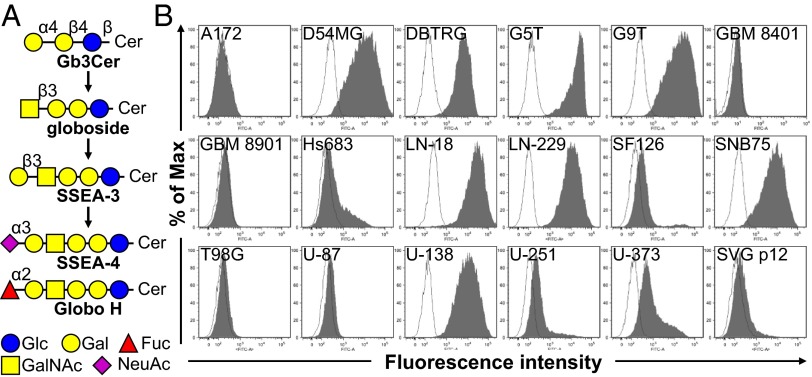
We analyzed the expression levels of various glycan epitopes by flow cytometry in four human GBM cell lines: G5T, LN-18, U-138, and U-251. The glycan epitopes examined include O-linked glycans [Tn, sTn, and Thomsen–Friedenreich (TF) antigens], Lewis antigens (Lex, Ley, and sLex), complex gangliosides [GM2, GM1, GD1a, GD2, GT1b, and A2B5 (c-series gangliosides)], and globo-series GSLs (SSEA-3, SSEA-4, and Globo H) (Fig. 1A). The results showed that most of these four GBM cell lines expressed high levels of Tn, TF, Lex, and Ley, a low level of sLex, and no sTn (Table 1 and Fig. S1 A and B). In addition, these four GBM cell lines were positive for all the gangliosides we examined, although the expression levels varied (Fig. S1C). U-251 showed weak MC813-70 (anti–SSEA-4) staining intensity, and G5T, U-138, and LN-18 displayed high MC813-70 staining intensity (Fig. 1B). Among these four cell lines, positive MC631 (anti–SSEA-3) staining was observed only on G5T, and none of the cell lines was positive for VK9 (anti-Globo H) staining (Table 1 and Fig. S1 D and E). After these findings, we looked further into the expression patterns of globo-series GSLs in additional GBM cell lines and found that of the nine of the 17 GBM cell lines showed strong MC813-70 staining signal (SSEA-4hi); three were weakly stained (SSEA-4lo), and five were not stained by MC813-70 (Fig. 1A). Nine of the 17 cell lines were positive for MC631 staining, and six were positive for VK9 staining (Fig. S1 D and E). SVG p12, an immortalized human fetal glia cell, showed a very weak MC813-70 staining signal and no MC631 nor VK-9 staining signal (Fig. 1 and Fig. S1). These results indicated that most of the GBM cell lines examined were positively stained by MC813-70.
Fig. 1.
The binding characteristics of mAb MC813-70 to GBM cell lines. (A) Schematic diagram of the biosynthesis of globo-series GSLs. SSEA-3, the precursor of SSEA-4 and Globo H, is synthesized from globoside. Glycosidic linkages and graphic notations are labeled. Glc, glucose; Gal, galactose; GalNAc, N-acetylgalactosamine; Fuc, fucose; NeuAc, N-acetylneuraminic acid. (B) GBM cells were stained with Alexa Fluor 488-conjugated MC813-70, and the staining intensity was analyzed with flow cytometry. All cells examined were GBM cell lines except for SVG p12, which is a normal human fetal glial cell line transformed with SV40 large T antigen. The histograms of the cells stained with MC813-70 and isotype control are shown in gray and white, respectively.
Table 1.
Expression profiles of glycan-related epitopes in GBM cell lines LN-18, U-138, U-251, and G5T
| Antigen | LN-18 | U-138 | U-251 | G5T |
| TF | 83.3 ± 3.4 | 76.1 ± 17.7 | 12.8 ± 11.7 | 5.8 ± 0.2 |
| Tn | 77.7 ± 4.3 | 49.9 ± 28.5 | 54.2 ± 18.2 | 20.7 ± 12.5 |
| sTn | 5.1 ± 0.3 | 10.9 ± 5.9 | 5.3 ± 0.5 | 4.9 ± 0.8 |
| Lex | 6.2 ± 0.7 | 22.2 ± 17.7 | 74.2 ± 14.6 | 76.5 ± 10.6 |
| Ley | 29.3 ± 9.9 | 46.0 ± 31.3 | 61.9 ± 21.8 | 8.8 ± 1.2 |
| sLex | 4.8 ± 0.5 | 32.8 ± 1.7 | 8.2 ± 5.2 | 5.4 ± 0.3 |
| GM2 | 82.8 ± 23.9 | 97.2 ± 2.7 | 89.8 ± 14.1 | 38.4 |
| GM1 | 98.5 ± 0.1 | 97.9 ± 1.6 | 74.6 ± 30.2 | 39.4 |
| GD1a | 96.9 ± 4.4 | 99.3 ± 1.0 | 88.0 ± 9.9 | 27.6 |
| GD2 | 43.4 ± 11.6 | 23.4 ± 9.7 | 10.3 ± 4.2 | 15.4 ± 8.9 |
| GT1b | 97.6 ± 3.3 | 96.1 ± 0.9 | 88.8 ± 1.5 | 30.1 ± 7.1 |
| A2B5 | 58.2 ± 41.1 | 59.2 ± 12.0 | 36.6 ± 18.0 | 21.1 ± 11.4 |
| SSEA-3 | 75.9 ± 16.2 | 38.9 ± 26.0 | 27.1 ± 24.8 | 97.7 ± 3.0 |
| SSEA-4 | 99.6 ± 0.2 | 90.5 ± 16.4 | 43.1 ± 10.8 | 99.9 ± 0.1 |
| Globo H | 22.5 | 11.8 | 9.3 ± 4.0 | 5.5 ± 0.1 |
Expression of glycan-related epitopes was determined by flow cytometry as described in Materials and Methods. Values are the percentage (mean ± SD) of positive cells in total cells.
Specificity of MC813-70.
Previous studies indicate that mAb MC813-70 recognizes the NeuAcα2-3Galβ1-3GalNAc epitope in SSEA-4 glycolipid (sialyl Gb5Cer) and the glycoproteins with an extended core 1 O-glycan structure (22, 27). In addition, MC813-70 shows cross-reactivity toward GM1b and GD1a when these two gangliosides are immobilized at a very high concentration/density. We used a glycan microarray (28) to investigate the binding specificity of MC813-70. As shown in Fig. 2, we found that, among the 152 chemically synthesized glycans on the glycan microarray (Fig. S2), MC813-70 recognizes only the SSEA-4 hexasaccharide with Neu5Ac or Neu5Gc at the terminal position (glycan Nos. 12 and 49). Compared with previous ELISA results (22), MC813-70 did not show any binding to GM1b (glycan No. 104) or GD1a (glycan No. 106), both of which contain the terminal trisaccharide epitope of SSEA-4 (NeuAcα2-3Galβ1-3GalNAc) with a different linkage (β1–4) to lactose at the reducing end. We also used the glycan array to determine the dissociation constants of MC813-70 with SSEA-4 hexasaccharide on the surface. The Kd value for the interaction was 4.21 ± 0.26 nM (Fig. 2, Inset), showing that MC813-70 is highly specific for SSEA-4.
Fig. 2.
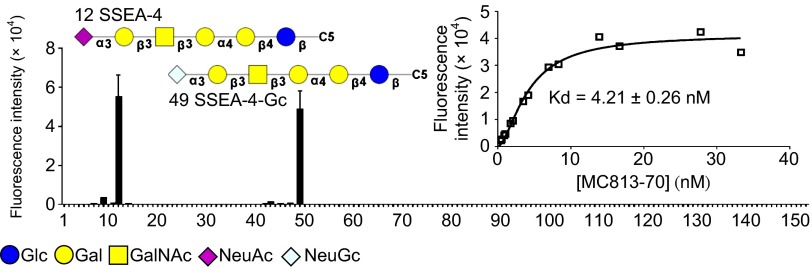
The glycan-binding profile of mAb MC813-70. The glycan microarrays on glass slides were probed with Alexa Flour 647-conjugated MC813-70 (10 μg/mL) and were read in an array scanner at 635 nm. Data are presented as mean ± SD. C5, C5H10NH2. (Inset) The binding curve of MC813-70 to SSEA-4. The dissociation constant (Kd) of mAb MC813-70 toward SSEA-4 was detected on a glass slide printed with SSEA-4 glycan.
Verification of SSEA-4 Expression on GBM Cell Lines.
To exclude the possibility that MC813-70 may bind to the extended core 1 O-glycan on glycoproteins in GBM cells, we treated DBTRG cells with methanol to remove lipids before staining with MC813-70. Upon methanol treatment, the immunoreactivity of MC813-70 disappeared, as analyzed by flow cytometry (Fig. S3A) and immunofluorescence microscopy (Fig. S3B), suggesting that MC813-70 is immunoreactive toward glycolipids, not glycoproteins. To confirm the presence of the SSEA-4 epitope on the GBM cell surface, we performed further MC631 staining on α2,3-sialidase–treated DBTRG cells (Fig. S4). When treated with α2,3-sialidase, the cells became MC813-70− and MC631+, indicating that the GBM cells did express SSEA-4.
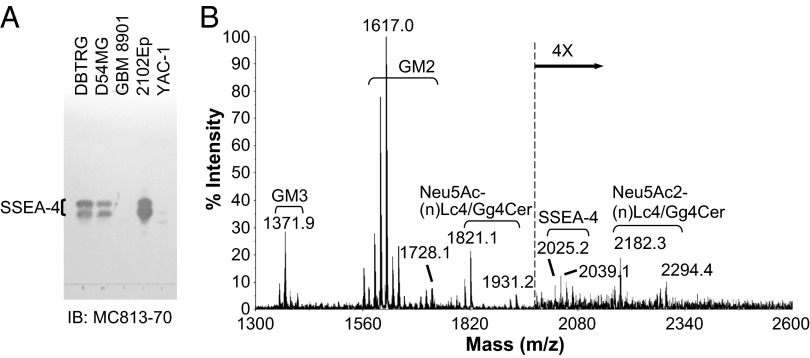
To verify the expression of SSEA-4 further, we next purified the gangliosides using anion-exchange chromatography, developed the purified gangliosides on HPTLC plates, and visualized them by orcinol-H2SO4 staining or immunoblotting. As shown in Fig. S5A, the purified gangliosides from three different GBM cell lines exhibited similar patterns (Fig. S5A, Left, lanes 1–3), with abundant GM3, GM2, Neu5Ac-(n)Lc4/Gg4Cer, and Neu5Ac2-(n)Lc4/Gg4Cer. Consistent with the results of flow cytometric analysis, MC813-70 recognized two gangliosides (because of a different chain length of fatty acids) on TLC from DBTRG and D54MG cells but not from GBM 8901 cells (Fig. 3A). The positions of the immunoreactive double bands in GBM gangliosides were the same as in the gangliosides purified from 2102Ep cells, which are embryonal carcinoma cells known to express a high level of SSEA-4 glycolipid (Fig. 3A, lane 4) (22). A double band that developed at a shorter distance than the MC813-70+ glycolipid was detected by MC813-70 in YAC-1 cells (Fig. 3A, lane 5), in which GM1b is a major ganglioside (29), indicating that MC813-70 harbors a weak cross-reactivity toward GM1b. Immunoblotting with MC631 revealed that it also could recognize MC813-70+ glycolipid but with a lower affinity than MC813-70 (Fig. S5A, Right). To examine the number of sialic acids on MC813-70+ glycolipids, we eluted gangliosides in different salt conditions and performed immunoblotting with MC813-70. The result indicated that MC813-70–reactive gangliosides were monosialylated, because the two bands appeared at the fraction eluted in the low-salt condition (Fig. S5B). We next used sialidases to elucidate the linkage of the sialic acid on this MC813-70–reactive monosialoganglioside. Gangliosides developed on a TLC plate were treated with α2,3-sialidase, the sialidase that cleaves all linkages of sialic acids, and were blotted with MC813-70 and MC631. The results in Fig. S5C show that the immunoreactivity of MC813-70 disappeared after sialidase treatment (Fig. S5C, Center), whereas MC631 could detect strong signals (dashed rectangle in Fig. S5C, Right) at the positions resembling MC813-70–reactive doublets, indicating the presence of an α2,3-linked sialic acid.
Fig. 3.
HPTLC immunostaining and MALDI-MS profiles of gangliosides from GBM cell lines. (A) Gangliosides were separated on an HPTLC plate and detected with MC813-70 mAb. Gangliosides from 2012Ep (a human embryonal carcinoma cell line) and YAC-1 (a mouse lymphoma cell line) served as the positive controls for SSEA-4 and GM1b, respectively. SSEA-4 with different chain lengths of fatty acids migrated as two close bands. (B) The extracted gangliosides from DBTRG GBM cells were permethylated and analyzed by MALDI-MS. The major gangliosides in DBTRG cells were GM3 (m/z = 1,371.9), GM2 (m/z = 1,617.0), Neu5Ac-(n)Lc4/Gg4Cer (m/z = 1,821.1), and Neu5Ac2-(n)Lc4/Gg4Cer (m/z = 2,182.3). SSEA-4 (Neu5Ac-Hex4-HexNAc-Cer, m/z = 2025.2) was also observed, albeit in a relatively weak signal. Gangliosides with the same glycan moiety but with different fatty acyl contents are bracketed.
We also analyzed the gangliosides from DBTRG cells by MALDI-MS profiling (Fig. 3B). The spectra were dominated by several major peaks that occurred in signal clusters because of the expected heterogeneity of the ceramide (Cer) portions. The respective gangliosides profiles were assigned based on the m/z values of major molecular ions, as fitted to permethylation of hexose (Hex), N-acetylhexosamine (HexNAc), or NeuAc residues, in combination with sphingosine and fatty acyl chains,. Consistent with the HPTLC results, MS profiling showed that the major species of gangliosides in DBTRG cells were GM3, GM2, Neu5Ac-(n)Lc4/Gg4Cer, and Neu5Ac2-(n)Lc4/Gg4Cer. The signal with Neu5Ac-Hex4-HexNAc-Cer (m/z = 2025.2) that represented SSEA-4 was detected also, although with low intensity, reflecting the existence of SSEA-4 in DBTRG cells. These data indicate that the MC813-70–reactive ganglioside was SSEA-4 and that, although it was a minor constituent of total gangliosides, SSEA-4 was expressed in GBM cells.
Expression of SSEA-4 in GBM Tissues.
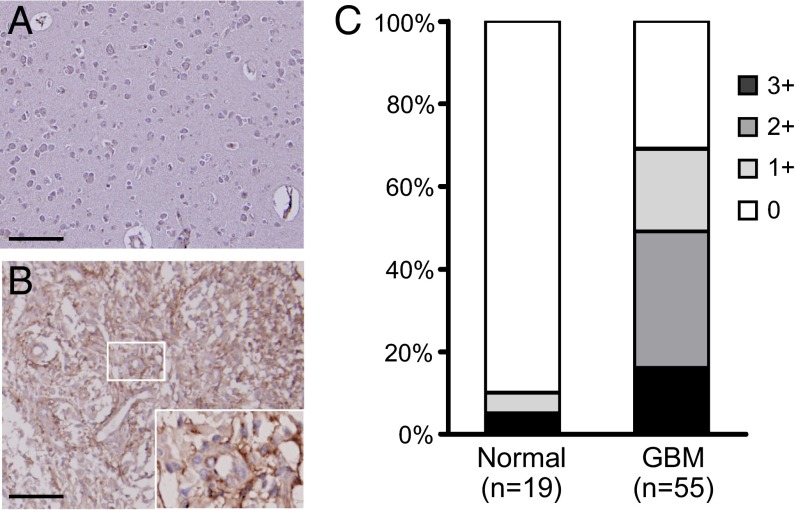
SSEA-4 is a widely used marker for stem cells, but information about the expression of SSEA-4 in GBM tissues as well as normal brain tissues has been limited. To understand if SSEA-4 is overexpressed in clinical GBM specimens, in addition to GBM cell lines, we analyzed the expression of SSEA-4 in grade I– IV astrocytomas and in normal brain tissues by immunohistochemistry (IHC) on human tissue microarrays (Fig. 4 and Fig. S6). We found that 38 of 55 GBM tissue specimens (69%) were positive for MC813-70 staining and around half of the GBM specimens were intensely stained, with a score of 2+ or higher (Fig. 4C and Fig. S6A). As shown in the positive specimens, SSEA-4 was situated on the plasma membrane of GBM cells (Fig. 4B, Inset). Furthermore, around 55% of low-grade astrocytoma specimens were weakly stained (scored as 1+) by MC813-70, and the SSEA-4 intensity scores correlated positively with astrocytoma grade (Fig. S6B). Most normal brain tissues were SSEA-4− (Fig. 4A). These results indicated SSEA-4 is highly expressed in GBM tumors.
Fig. 4.
Expression of SSEA-4 in GBM. (A and B) Representative images of normal brain tissues (A) and GBM (B) after immunohistochemical staining with MC-813–70. The inset in B shows a magnified picture of the small boxed area. (Scale bars, 100 μm.) (C) Statistical results of SSEA-4 IHC. GBM specimens (n = 55) and normal brain tissues (n = 19) were counterstained with hematoxylin after IHC. The staining intensity of the tissues was graded as 0 (negative), 1+ (weak), 2+ (moderate), and 3+ (strong).
MC813-70 Mediates Complement-Dependent Cytotoxicity Against GBM Cell Lines.
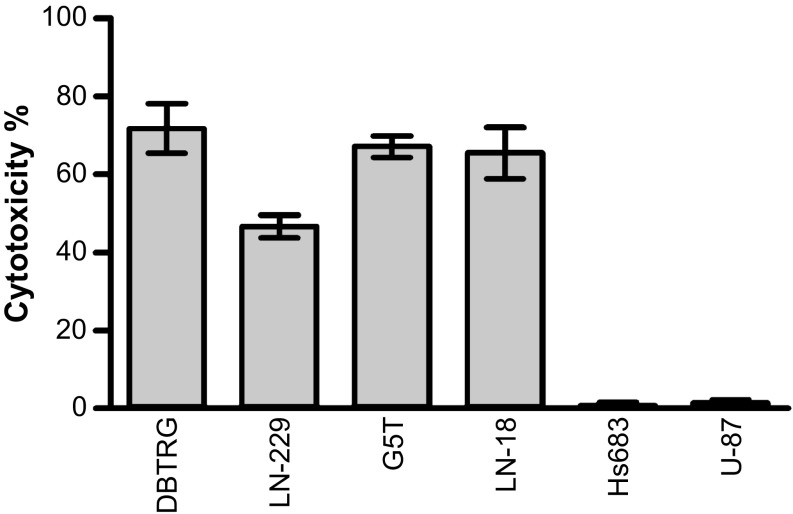
To test if targeting SSEA-4 triggers complement-dependent cytotoxicity (CDC) in GBM cells, GBM cell lines were treated with MC813-70 and rabbit complement, and the degree of CDC was evaluated by detecting the level of released lactate dehydrogenase (LDH) caused by cell death. Fig. 5 shows that, in the presence of complement, mAb MC813-70 remarkably reduced the number of viable GBM cells. We observed a significant CDC in SSEA-4hi GBM cell lines: 71.7% cytotoxicity of DBTRG, 46.6% of LN-229, 67% of G5T, and 65.4% of LN-18 cells. MC813-70–mediated CDC did not kill two GBM cell lines, Hs683 and U87, that expressed low or no SSEA-4. Therefore, the level of MC813-70–mediated CDC correlated positively with the level of SSEA-4expression in each GBM cell line.
Fig. 5.
MC813-70 triggered CDC in GBM cells. GBM cell lines were treated with 20 µg/mL MC813-70 and rabbit complement to observe MC813-70–induced cell lysis. The CDC activity of MC813-70 was measured by the LDH release assay as described in Materials and Methods. The data are shown as mean ± SD.
MC813-70 Suppresses Brain Tumor Growth in Vivo.
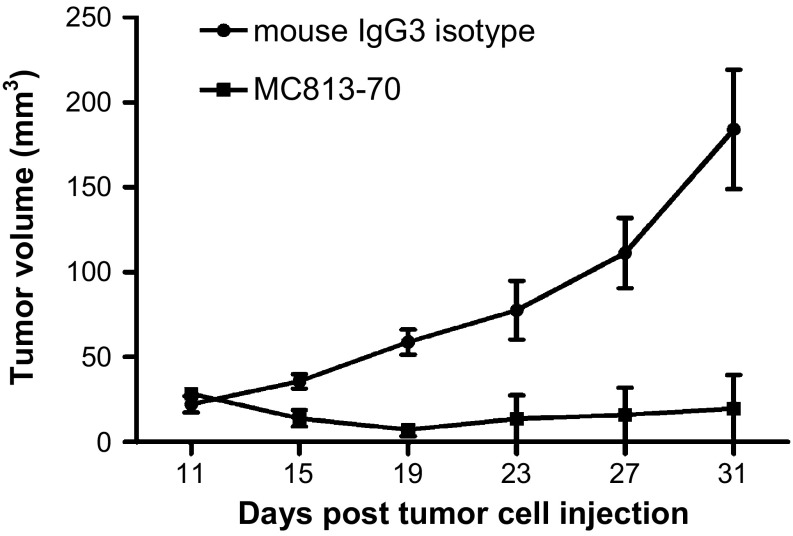
To check if MC813-70 was able to suppress GBM tumor growth in vivo, MC813-70 was administered to the nude mice injected s.c. with DBTRG cells when the tumors grew to palpable bumps (15–30 mm3 at day 11 postinjection). MC813-70 (200 μg) was given i.p. to each mouse every 4 d for a total of three times; an irrelevant mouse IgG3 (isotype control) was injected in parallel for comparison. The experiment revealed that the administration of MC813-70 could inhibit tumor growth in DBTRG cells (Fig. 6). The growth of DBTRG tumors was completely suppressed in two of three mice treated with MC813-70; the third mouse developed tumor after the cessation of antibody treatment. As compared with mice receiving MC813-70 treatment, DBTRG tumors grew aggressively (with an average tumor volume of 184 mm3 at day 31) in the control group injected with mouse IgG3. These results demonstrated that MC813-70 can inhibit the growth of SSEA-4–expressing GBM tumors, possibly through CDC and antibody-dependent cell-mediated cytotoxicity in vivo.
Fig. 6.
Inhibition of DBTRG tumor growth by MC813-70. On day 0, male nude mice were inoculated with DBTRG cells in the right flank, and MC813-70 or mouse IgG3 isotype control (200 µg per dose) was administered i.p. at days 11, 15, and 19. Mice were killed at day 31. The tumor volume in each group (n = 3) was measured at different time points and is shown as mean ± SD. P = 0.001 was obtained by two-way ANOVA.
Expression of SSEA-4 in Various Cancer Cell Lines.
SSEA-4 expression has been reported on renal carcinoma (26), basaloid lung cancer (30), epithelial ovarian carcinoma (31), breast cancer (25), and oral cancer (32) cells. Here, we analyzed the expression levels of SSEA-3, Globo H, and SSEA-4 on various cancer cell lines by flow cytometry. As shown in Table 2, we examined 134 cancer cell lines (17 brain cancer cell lines, 20 lung cancer cell lines, 23 breast cancer cell lines, 13 oral cancer cell lines, two esophageal cancer cell lines, six gastric cancer cell lines, 10 liver cancer cell lines, five bile duct cancer cell lines, eight pancreatic cancer cell lines, seven colon cancer cell lines, six renal cancer cell lines, four cervical cancer cell lines, nine ovarian cancer cell lines, and four prostate cancer cell lines). The names and the expressed GSLs of these cell lines are listed in Table S1. We found that SSEA-4 was expressed in every type of cancer cell line (in 96 of 134 cancer cell lines). Globo H was expressed in 88 of 134 cancer cell lines, preferentially in lung, breast, pancreas, colon, stomach, mouth, liver, and kidney cancer cell lines, and 70 of 134 cancer cell lines expressed SSEA-4 and Globo H simultaneously. On the other hand, SSEA-3 expression was always accompanied by a high level of SSEA-4 expression, indicating that SSEA-4 and Globo H are the major globo-series GSLs on the cancer cells. To validate the identity of SSEA-4, we purified the gangliosides from nine MC813-70+ cell lines and from TF1a, an MC813-70− leukemia cell line, and performed immunostaining on HPTLC plates. As expected, SSEA-4 was detected in these nine cancer cell lines but not in TF1a (Fig. S7), and the intensity correlated well with the geometric mean fluorescence intensity as examined by flow cytometry. These results showed that SSEA-4 can be expressed in a variety of cancer cell lines and may serve as a potential target for multiple types of cancers.
Table 2.
Expression of globo-series GSLs in cancer cell lines
| Tumor origin | SSEA-4+ | SSEA-3+ | Globo H+ | SSEA4+ or SSEA3+ or Globo H+ | SSEA-4+ SSEA-3+ | SSEA-4+ Globo H+ | SSEA-4+ SSEA-3+ Globo H+ |
| Brain | 12/17 | 9/17 | 6/17 | 12/17 | 9/17 | 6/17 | 5/17 |
| Lung | 13/20 | 5/20 | 13/20 | 16/20 | 5/20 | 10/20 | 5/20 |
| Breast | 17/23 | 6/23 | 14/23 | 18/23 | 6/23 | 13/23 | 6/23 |
| Mouth | 8/13 | 2/13 | 11/13 | 12/13 | 2/13 | 7/13 | 2/13 |
| Esophagus | 1/2 | 0/2 | 2/2 | 2/2 | 0/2 | 1/2 | 0/2 |
| Stomach | 4/6 | 3/6 | 6/6 | 6/6 | 3/6 | 4/6 | 3/6 |
| Liver | 6/10 | 4/10 | 9/10 | 9/10 | 4/10 | 6/10 | 4/10 |
| Bile duct | 2/5 | 1/5 | 3/5 | 3/5 | 1/5 | 2/5 | 1/5 |
| Pancreas | 8/8 | 3/8 | 6/8 | 8/8 | 3/8 | 6/8 | 3/8 |
| Colon | 5/7 | 0/7 | 6/7 | 7/7 | 0/7 | 4/7 | 0/7 |
| Kidney | 5/6 | 0/6 | 5/6 | 6/6 | 0/6 | 4/6 | 0/6 |
| Cervix | 3/4 | 2/4 | 1/4 | 3/4 | 2/4 | 1/4 | 0/4 |
| Ovary | 8/9 | 2/9 | 5/9 | 8/9 | 2/9 | 5/9 | 2/9 |
| Prostate | 4/4 | 1/4 | 1/4 | 4/4 | 1/4 | 1/4 | 0/4 |
Expression of globo-series GSLs was determined by flow cytometry. Cell lines in which more than 15% of total cells were positive in flow cytometry are labeled positive.
Discussion
In the present study, we evaluated the expression of globo-series GSLs on human GBM cell lines and found that SSEA-4 is highly expressed in most GBM cell lines; SSEA-3 and Globo H also are expressed at lower levels.
SSEA-4, first identified in 1983 (22), often has been used as a pluripotency marker for hESCs and for the isolation and characterization of cells with stem cell properties. Nonetheless, information about the distribution of SSEA-4 in normal tissues is limited. SSEA-4 has been reported to be expressed as a minor GSL in erythrocytes (22) and on the epithelial cells of several glandular tissues [e.g., breast, colon, gastrointestinal tract, kidney, lung, ovary, pancreas, rectum, stomach, testes, thymus, and uterine cervix (24)]. In solid tumors, SSEA-4 expression was found on renal cell carcinomas in 1997 by Saito et al. (26) using IHC with RM1, another SSEA-4 mAb. In the last few years, SSEA-4 expression has been reported on breast cancer cells and breast cancer stem cells (25), basaloid lung cancer (30), epithelial ovarian carcinoma (31), and oral cancer (32). The relationship between the level of SSEA-4 expression and tumor malignancy differs in various types of cancers: The expression of SSEA-4 in basaloid lung cancer is associated with poor prognosis (30), whereas a reduction of SSEA-4 correlates with more advanced tumor stage and tumor cell differentiation in ovarian cancer (31). Here, we examined the expression of SSEA-4 in GBM and found that SSEA-4 is expressed not only in GBM/grade IV astrocytoma but also in ∼55% of grade I, ∼55% of grade II, and ∼60% of grade III astrocytoma. Nevertheless, there appears to be a trend for higher expression of SSEA-4 to be associated with higher grade of astrocytomas. We propose that SSEA-4 may play an active role during tumor progression of astrocytomas and may serve as a potential therapeutic target in patients with GBM as well as low-grade astrocytomas. Furthermore, we provide evidence that SSEA-4 is expressed in multiple cancer cell lines, including lung, breast, ovarian, prostate, colon, and pancreatic cancers.
Although SSEA-4 has been known for more than 20 y, the molecular function of SSEA-4 has not been tested experimentally. Brimble et al. (33) depleted SSEA-3 and -4 in hESCs with d-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol (d-PDMP), an inhibitor for glucosylceramide synthase, and found that d-PDMP treatment did not alter the gene-expression profile of hESCs or alter their capacity to differentiate in vitro, suggesting that SSEA-4 is not essential for hESCs’ pluripotency. On the other hand, Van Slambrouck et al. (34) demonstrated that clustering of monosialyl-Gb5 induces the invasion of MCF-7 breast cancer cells by activating the FAK/c-Src signaling pathway. Recently, SSEA-4 was reported to bind to FK-506–binding protein 4, which may affect the transportation of SSEA-4 to the cell surface and the downstream signaling pathway (35).
In addition to SSEA-4, we examined the expression of two other globo-series GSLs, SSEA-3 and Globo H. SSEA-3 was expressed mainly in cell lines with high SSEA-4 expression. This result, together with the observation of Globo H expression in only six of the GBM cell lines we tested, indicates that SSEA-4 is the major globo-series GSL in GBM and that, once SSEA-3 is synthesized, it is converted efficiently to SSEA-4 (24). It has been suggested that an α2,3-sialyltransferase, encoded by ST3GAL2 gene, is the major SSEA-4 synthase that is closely related to renal carcinogenesis (36) and that the mRNA level of ST3GAL2 is increased in renal and colorectal carcinomas (37). We also observed that the mRNA level of ST3GAL2, but not of fucosyltransferase (FUT) 1 and 2, is abundant in GBM cell lines (Fig. S8), perhaps explaining why SSEA-4 is the predominant globo-series GSL in GBM cells. It is also interesting to establish the correlation of A4GALT, ST3GAL2, FUT1, and FUT2 mRNA levels with the expression of globo-series GSLs in GBM specimens, to help detect globo-series GSLs in small amounts of tumor samples.
Targeted therapy, which blocks tumor growth by using small molecules or mAbs to interfere with specific molecules, is a growing part of many cancer treatment regimens (38); for example, a series of anti-EGFRvIII mAbs has been generated for GBM therapy (39, 40). Moreover, anti-glycolipid–targeted therapy is showing great promise in the treatment of several cancers (41) [e.g., anti-GD2 in neuroblastoma (42)]. In addition, a recent report indicates that a Globo H-DT vaccine can elicit specific IgG not only against Globo H but also against SSEA-3 and SSEA-4 (25). The expression profile of these three glycolipids in different types of cancer cell lines, as shown in Table 2, provides a direction in cancer vaccine therapy. In addition, our finding of high-level SSEA-4 expression in GBM provides a potential target for GBM diagnosis and therapy.
Materials and Methods
For additional details, see SI Materials and Methods.
Flow Cytometry.
Cells (1 × 105) were stained with 0.5 μg Alexa Flour 488-conjugated anti–SSEA-3 mAb (MC-631), anti–SSEA-4 mAb (MC813-70), or allophycocyanin (APC)-conjugated anti-Globo H mAb (VK9, a gift from Philip O. Livingston, Memorial Sloan–Kettering Cancer Center, New York) in 50 μL FACS buffer (PBS solution with 1% FBS) on ice for 30 min. For lectin staining, cells were incubated for 30 min on ice in lectin-binding buffer [1% BSA, 0.5× Carbo-Free Blocking buffer (Vector Laboratories), 2 mM MgCl2, 2 mM CaCl2] containing biotinylated lectin. After being washed twice with lectin-binding buffer, cells were incubated with streptavidin-APC (1:500 diluted in FACS buffer; Biolegend). After being washed twice with 200 μL FACS buffer, cells were resuspended in 200 μL FACS buffer containing 1 μg/mL propidium iodide (PI) and subjected to analysis. Data acquisition was performed on a FACSCanto (BD Biosciences) with FACSDiva software (BD Biosciences), and data analyses were performed using FlowJo software (TreeStar). Live PI− cells were gated for analysis. For methanol washing, cells were washed and fixed with 4% (wt/vol) paraformaldehyde in PBS for 15 min at room temperature, followed by incubation in methanol for 10 min before staining with specific antibodies.
TLC Immunostaining.
GSLs were separated on HPTLC plates as described above. After chromatography, the TLC plate was air-dried, immersed three times in 2.1% (wt/vol) poly(isobutyl-methacrylate) (Sigma) in hexane/chloroform (42:8, vol/vol), and soaked overnight in PBS at 37 °C. The plate was dried, blocked with in PBS for 30 min at room temperature, and reacted with MC813-70 or MC-631 (5 μg/mL) for 2 h at room temperature. After being gently washed three times with PBS and 0.05% Tween-20 (PBST), the plate was incubated with biotinylated secondary antibody (1 μg/mL) for 1 h, followed by incubation with streptavidin-alkaline phosphatase (1:1,000; Millipore). After washing with PBST, the TLC plate was developed with nitro blue tetrazolium/(5-bromo-4-chloro-1H-indol-3-yl) dihydrogen phosphate (Thermo Scientific).
In Vivo Tumor Growth.
BALB/cAnN.Cg-Foxn1nu/CrlNarl mice were purchased from the National Laboratory Animal Center (Taiwan) and maintained under specific pathogen-free conditions. The animals’ health status was monitored daily. Procedures involving animals and their care were conducted according to the guidelines of the Academia Sinica Institutional Animal Care and Utilization Committee in compliance with national and international laws and policies. DBTRG cells (1 × 107/250 μL PBS) were s.c. injected into the flank regions of 8- to 10-wk-old mice to generate the xenograft model. On days 11, 15, and 19, each mouse was i.p. injected with 200 μg of MC813-70 (purified from the ascites) or with mouse IgG3 isotype as the control antibody. Tumor size was determined by Vernier caliper by measuring the length (L) and width (W), and the tumor volume (in mm3) was calculated as 1/2 × LW2.
Supplementary Material
Acknowledgments
We thank Drs. Jin-Yuh Shew, Hua-Chien Chen, Chia-Ning Shen, and Shih-Hwa Chiou for providing various cancer cell lines and the Genomics Research Center MS Core Facilities, Academia Sinica, for glycolipid analysis. We are also grateful to the experimental assistance from Dr. Chien-Yu Chen and Tsung-Ching Lai. This research was supported by the Genomics Research Center, Academia Sinica, Taiwan.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1400283111/-/DCSupplemental.
References
- 1.Louis DN, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Meir EG, et al. Exciting new advances in neuro-oncology: The avenue to a cure for malignant glioma. CA Cancer J Clin. 2010;60(3):166–193. doi: 10.3322/caac.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyer MA. Malignant gliomas in adults. N Engl J Med. 2008;359(17):1850, author reply 1850. doi: 10.1056/NEJMc086380. [DOI] [PubMed] [Google Scholar]
- 4.Meezan E, Wu HC, Black PH, Robbins PW. Comparative studies on the carbohydrate-containing membrane components of normal and virus-transformed mouse fibroblasts. II. Separation of glycoproteins and glycopeptides by sephadex chromatography. Biochemistry. 1969;8(6):2518–2524. doi: 10.1021/bi00834a039. [DOI] [PubMed] [Google Scholar]
- 5.Hakomori S. Glycosylation defining cancer malignancy: New wine in an old bottle. Proc Natl Acad Sci USA. 2002;99(16):10231–10233. doi: 10.1073/pnas.172380699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lau KS, Dennis JW. N-Glycans in cancer progression. Glycobiology. 2008;18(10):750–760. doi: 10.1093/glycob/cwn071. [DOI] [PubMed] [Google Scholar]
- 7.van Beek WP, Smets LA, Emmelot P. Increased sialic acid density in surface glycoprotein of transformed and malignant cells—a general phenomenon? Cancer Res. 1973;33(11):2913–2922. [PubMed] [Google Scholar]
- 8.Sell S. Cancer-associated carbohydrates identified by monoclonal antibodies. Hum Pathol. 1990;21(10):1003–1019. doi: 10.1016/0046-8177(90)90250-9. [DOI] [PubMed] [Google Scholar]
- 9.Hakomori S, Zhang Y. Glycosphingolipid antigens and cancer therapy. Chem Biol. 1997;4(2):97–104. doi: 10.1016/s1074-5521(97)90253-2. [DOI] [PubMed] [Google Scholar]
- 10.Taylor-Papadimitriou J, Epenetos AA. Exploiting altered glycosylation patterns in cancer: Progress and challenges in diagnosis and therapy. Trends Biotechnol. 1994;12(6):227–233. doi: 10.1016/0167-7799(94)90121-X. [DOI] [PubMed] [Google Scholar]
- 11.Birklé S, Zeng G, Gao L, Yu RK, Aubry J. Role of tumor-associated gangliosides in cancer progression. Biochimie. 2003;85(3-4):455–463. doi: 10.1016/s0300-9084(03)00006-3. [DOI] [PubMed] [Google Scholar]
- 12.Fredman P, et al. Potential ganglioside antigens associated with human gliomas. Neurol Res. 1986;8(2):123–126. doi: 10.1080/01616412.1986.11739744. [DOI] [PubMed] [Google Scholar]
- 13.Yates AJ, Becker LE, Sachs LA. Brain tumors in childhood. Childs Brain. 1979;5(1):31–39. doi: 10.1159/000119799. [DOI] [PubMed] [Google Scholar]
- 14.Traylor TD, Hogan EL. Gangliosides of human cerebral astrocytomas. J Neurochem. 1980;34(1):126–131. doi: 10.1111/j.1471-4159.1980.tb04630.x. [DOI] [PubMed] [Google Scholar]
- 15.Berra B, Gaini SM, Riboni L. Correlation between ganglioside distribution and histological grading of human astrocytomas. Int J Cancer. 1985;36(3):363–366. [PubMed] [Google Scholar]
- 16.Fredman P, von Holst H, Collins VP, Granholm L, Svennerholm L. Sialyllactotetraosylceramide, a ganglioside marker for human malignant gliomas. J Neurochem. 1988;50(3):912–919. doi: 10.1111/j.1471-4159.1988.tb02999.x. [DOI] [PubMed] [Google Scholar]
- 17.Månsson JE, et al. Characterization of new gangliosides of the lactotetraose series in murine xenografts of a human glioma cell line. FEBS Lett. 1986;201(1):109–113. doi: 10.1016/0014-5793(86)80580-4. [DOI] [PubMed] [Google Scholar]
- 18.Fredman P, von Holst H, Collins VP, Dellheden B, Svennerholm L. Expression of gangliosides GD3 and 3′-isoLM1 in autopsy brains from patients with malignant tumors. J Neurochem. 1993;60(1):99–105. doi: 10.1111/j.1471-4159.1993.tb05827.x. [DOI] [PubMed] [Google Scholar]
- 19.Svennerholm L, et al. Human brain gangliosides: Developmental changes from early fetal stage to advanced age. Biochim Biophys Acta. 1989;1005(2):109–117. doi: 10.1016/0005-2760(89)90175-6. [DOI] [PubMed] [Google Scholar]
- 20.Kato Y, et al. GMab-1, a high-affinity anti-3′-isoLM1/3′,6′-isoLD1 IgG monoclonal antibody, raised in lacto-series ganglioside-defective knockout mice. Biochem Biophys Res Commun. 2010;391(1):750–755. doi: 10.1016/j.bbrc.2009.11.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schenkel-Brunner H. P System. Human Blood Groups. Vienna: Springer; 1995. pp. 211–234. [Google Scholar]
- 22.Kannagi R, et al. Stage-specific embryonic antigens (SSEA-3 and -4) are epitopes of a unique globo-series ganglioside isolated from human teratocarcinoma cells. EMBO J. 1983;2(12):2355–2361. doi: 10.1002/j.1460-2075.1983.tb01746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang S, et al. Selection of tumor antigens as targets for immune attack using immunohistochemistry: I. Focus on gangliosides. Int J Cancer. 1997;73(1):42–49. doi: 10.1002/(sici)1097-0215(19970926)73:1<42::aid-ijc8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 24.Chang WW, et al. Expression of Globo H and SSEA3 in breast cancer stem cells and the involvement of fucosyl transferases 1 and 2 in Globo H synthesis. Proc Natl Acad Sci USA. 2008;105(33):11667–11672. doi: 10.1073/pnas.0804979105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang YL, et al. Carbohydrate-based vaccines with a glycolipid adjuvant for breast cancer. Proc Natl Acad Sci USA. 2013;110(7):2517–2522. doi: 10.1073/pnas.1222649110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saito S, et al. Expression of globo-series gangliosides in human renal cell carcinoma. Jpn J Cancer Res. 1997;88(7):652–659. doi: 10.1111/j.1349-7006.1997.tb00433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kannagi R, et al. New globoseries glycosphingolipids in human teratocarcinoma reactive with the monoclonal antibody directed to a developmentally regulated antigen, stage-specific embryonic antigen 3. J Biol Chem. 1983;258(14):8934–8942. [PubMed] [Google Scholar]
- 28.Wang CC, et al. Glycan microarray of Globo H and related structures for quantitative analysis of breast cancer. Proc Natl Acad Sci USA. 2008;105(33):11661–11666. doi: 10.1073/pnas.0804923105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zarei M, Müthing J, Peter-Katalinić J, Bindila L. Separation and identification of GM1b pathway Neu5Ac- and Neu5Gc gangliosides by on-line nanoHPLC-QToF MS and tandem MS: Toward glycolipidomics screening of animal cell lines. Glycobiology. 2010;20(1):118–126. doi: 10.1093/glycob/cwp154. [DOI] [PubMed] [Google Scholar]
- 30.Gottschling S, et al. Stage-specific embryonic antigen-4 is expressed in basaloid lung cancer and associated with poor prognosis. Eur Respir J. 2013;41(3):656–663. doi: 10.1183/09031936.00225711. [DOI] [PubMed] [Google Scholar]
- 31.Ye F, et al. Stage-specific embryonic antigen 4 expression in epithelial ovarian carcinoma. Int J Gynecol Cancer. 2010;20(6):958–964. doi: 10.1111/IGC.0b013e3181e6fee1. [DOI] [PubMed] [Google Scholar]
- 32.Noto Z, et al. CD44 and SSEA-4 positive cells in an oral cancer cell line HSC-4 possess cancer stem-like cell characteristics. Oral Oncol. 2013;49(8):787–795. doi: 10.1016/j.oraloncology.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 33.Brimble SN, et al. The cell surface glycosphingolipids SSEA-3 and SSEA-4 are not essential for human ESC pluripotency. Stem Cells. 2007;25(1):54–62. doi: 10.1634/stemcells.2006-0232. [DOI] [PubMed] [Google Scholar]
- 34.Van Slambrouck S, Steelant WF. Clustering of monosialyl-Gb5 initiates downstream signalling events leading to invasion of MCF-7 breast cancer cells. Biochem J. 2007;401(3):689–699. doi: 10.1042/BJ20060944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hung TC, Lin CW, Hsu TL, Wu CY, Wong CH. Investigation of SSEA-4 binding protein in breast cancer cells. J Am Chem Soc. 2013;135(16):5934–5937. doi: 10.1021/ja312210c. [DOI] [PubMed] [Google Scholar]
- 36.Saito S, et al. Human alpha2,3-sialyltransferase (ST3Gal II) is a stage-specific embryonic antigen-4 synthase. J Biol Chem. 2003;278(29):26474–26479. doi: 10.1074/jbc.M213223200. [DOI] [PubMed] [Google Scholar]
- 37.Kudo T, et al. Up-regulation of a set of glycosyltransferase genes in human colorectal cancer. Lab Invest. 1998;78(7):797–811. [PubMed] [Google Scholar]
- 38.Green MR. Targeting targeted therapy. N Engl J Med. 2004;350(21):2191–2193. doi: 10.1056/NEJMe048101. [DOI] [PubMed] [Google Scholar]
- 39.Mishima K, et al. Growth suppression of intracranial xenografted glioblastomas overexpressing mutant epidermal growth factor receptors by systemic administration of monoclonal antibody (mAb) 806, a novel monoclonal antibody directed to the receptor. Cancer Res. 2001;61(14):5349–5354. [PubMed] [Google Scholar]
- 40.Wikstrand CJ, Cokgor I, Sampson JH, Bigner DD. Monoclonal antibody therapy of human gliomas: Current status and future approaches. Cancer Metastasis Rev. 1999;18(4):451–464. doi: 10.1023/a:1006354102377. [DOI] [PubMed] [Google Scholar]
- 41.Durrant LG, Noble P, Spendlove I. Immunology in the clinic review series; focus on cancer: Glycolipids as targets for tumour immunotherapy. Clin Exp Immunol. 2012;167(2):206–215. doi: 10.1111/j.1365-2249.2011.04516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu AL, et al. Children’s Oncology Group Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363(14):1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.