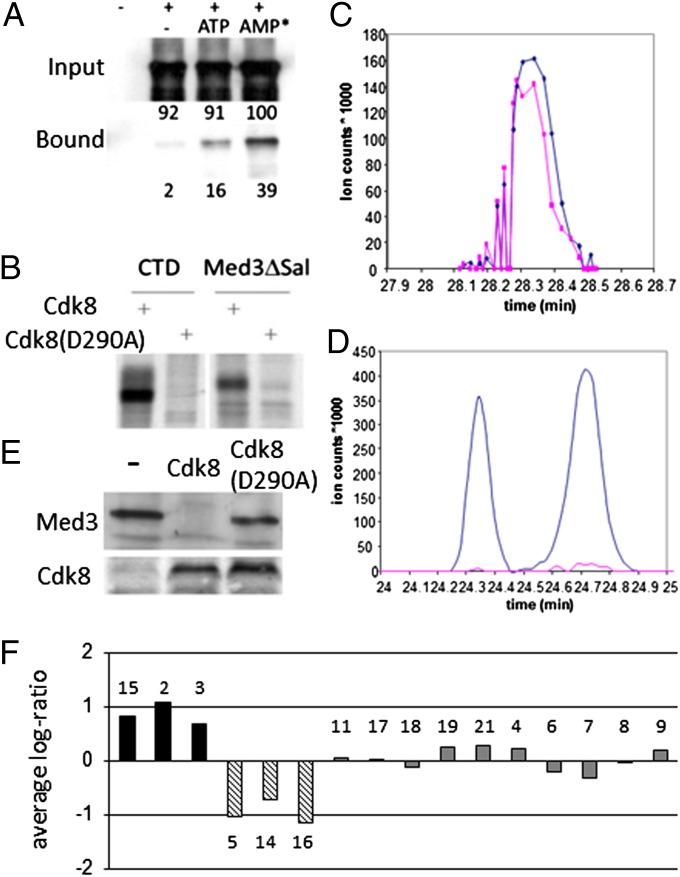
Fig. 2.
Med3 interacts with and is phosphorylated by Cdk8. (A) Recombinant Med3ΔSal protein interacts with myc-Cdk8, and this interaction is enhanced in the presence of ATP and AMPNP (AMP*). Purified myc-Cdk8 was incubated with Med3ΔSal protein and either 500 mM ATP or AMPNP. Values indicate percentage protein relative to AMPNP input reaction. (B) Cdk8 phosphorylates Med3. Recombinant histidine-tagged RNA pol II CTD or Med3ΔSal protein was incubated with myc-Cdk8 in the presence of 33P γ-ATP. Extracted ion chromatograms of (C) nonphosphorylated or (D) phosphorylated Med3-peptide SGSTMGTPTVHNSTAAAPIAAPK obtained from WT (blue) and cdk8Δ (pink) strains. (E) Med3 is unstable in the presence of Cdk8 in vivo. Total protein isolated from cells transformed with MED3 and either CDK8 or cdk8 (D290A) were analyzed for Med3 or Cdk8 by immunoblotting with anti-HA or anti-myc, respectively. (F) Mediator stability in absence of Cdk8. Quantitative MS analysis of Tap-Med15 purified Med proteins in the presence or absence of Cdk8. Observed ratios of purified Med proteins (numbered) were normalized so that the average log-ratio equaled 0 between samples.