Abstract
Background
Universal immunization of adolescents against meningococcal disease with a quadrivalent meningococcal ACWY (MenACWY) conjugate vaccine is recommended in a number of countries.
Methods
In a randomized, controlled, observer-blinded, multicenter trial, 1016 participants, 10–25 years of age, were randomly allocated 1:1:1 to receive a single dose of 1 of 2 lots of an investigational tetanus toxoid‐conjugated MenACWY vaccine (MenACWY‐TT) or a marketed diphtheria toxoid‐conjugated MenACWY vaccine (MenACWY‐DT). The primary outcome was the noninferiority of the vaccine response after MenACWY‐TT (lot A) compared with MenACWY‐DT for all 4 serogroups. Vaccine response was defined as a postvaccination human serum bactericidal antibody (hSBA) titer against each of the serogroups of at least 1:8 in persons initially seronegative (<1:4) or as a 4‐fold increase in titer pre‐ to postvaccination in persons initially seropositive (≥1:4). Adverse events (AEs) after immunization were measured 4 and 31 days postvaccination.
Results
The mean age of participants was 16.3 years; 977 (96.6%) completed the study. The noninferiority of MenACWY‐TT (lot A) to the control vaccine in terms of the percentage of participants with hSBA vaccine response was demonstrated for each serogroup. Vaccine response rates ranged from 51.0% to 82.5% for the 4 serogroups after MenACWY‐TT (both lots) compared with 39.0%–76.3% for the 4 serogroups after MenACWY‐DT. Pain was the most common injection‐site reaction reported by 50.8%–55.4% across the 3 groups. Fatigue and headache were the most common systemic solicited AEs, reported by 27.3%–29.2% and 25.5%–26.4%, respectively.
Conclusions
Tetanus toxoid‐conjugated MenACWY vaccine was well tolerated and elicited an immune response that was noninferior to that of a marketed MenACWY‐DT (www.clinicaltrials.gov NCT01165242).
Keywords: meningococcal conjugate vaccine, Neisseria meningitidis, vaccine safety, vaccine immunogenicity
Neisseria meningitidis is an important cause of invasive bacterial infection worldwide [1]. Disease manifestations include bacteremia and sepsis, meningitis, septic arthritis, and pericarditis. Death and long-term sequelae including hearing loss, neurological disabilities, and limb loss in survivors are not uncommon after invasive meningococcal disease [2]. Although there are 12 immunologically distinct serogroups of N meningitidis, 6 serogroups (A, B, C, W-135, X, Y) cause most disease in humans [1, 2]. Polysaccharide vaccines against serogroups A, C, W-135, and Y have been available for decades, but, like other polysaccharide vaccines, they are limited in the durability of the protective responses, fail to reduce nasopharyngeal carriage, do not provide opportunities for boosting with subsequent doses, and, except for serogroup A, are not immunogenic in children under 2 years of age [3]. The development of conjugate vaccines, where the polysaccharide antigens are covalently linked to proteins, has led to more immunogenic vaccines for use in children for the prevention of several infectious diseases, including invasive meningococcal disease. Meningococcal C (MenC) conjugate vaccines have been approved in Europe, Canada, and elsewhere for use at 2 months of age and older beginning in 1999 [4]. Addition of MenC conjugate vaccines to the routine pediatric vaccination schedule has resulted in dramatic decreases in invasive MenC disease in many countries including Australia, the United Kingdom, the Netherlands, and Canada [5–9]. Two quadrivalent meningococcal conjugate vaccines using diphtheria toxoid or diphtheria toxoid cross-reacting material (CRM) as the conjugate protein (MenACWY-DT and MenACWY-CRM, respectively) have been developed and approved for use in individuals 9 months (MenACWY-DT) or 2 years (MenACWY-CRM) to 55 years of age, and they are now recommended for universal preadolescent and adolescent immunization in the United States and some provinces in Canada [10, 11]. The MenB capsular polysaccharide is poorly immunogenic because of antigenic similarities to human neural tissue, which has precluded development of MenB conjugate vaccines [12]. Therefore, broadly protective MenB vaccines are being developed using universally expressed N meningitidis surface proteins [13, 14].
Recently, a novel quadrivalent MenACWY conjugate vaccine using tetanus toxoid as the carrier protein (MenACWY-TT) has been developed and has undergone clinical trials in toddlers, adolescents, and adults [15–22]. The purpose of this study was to evaluate the safety and immunogenicity of MenACWY-TT compared with a marketed MenACWY-DT. Because the percentage of O-acetylation of the MenA polysaccharide may be important in its immunogenicity [23], 2 representative lots of MenACWY-TT covering the manufacturing range of O-acetylation of the MenA polysaccharide were compared.
METHODS
Study Design
This was a randomized, observer-blinded, multicentered, phase 2 clinical trial conducted in 33 centers in Canada and the United States between August 2010 and March 2011. Healthy individuals between 10 and 25 years of age were randomly allocated in a 1:1:1 ratio to 1 of 2 lots of MenACWY-TT or to MenACWY-DT. The study was conducted according to the International Conference on Harmonisation principles of Good Clinical Practice, the Declaration of Helsinki, and the Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans. The protocol received ethics approval at each participating center; written informed consent was obtained from all participants or their parent or legal guardian (ClinicalTrials.Gov NCT01165242).
Study Participants
Healthy males and females from 10 to 25 years of age, inclusive, were eligible to participate in the study. Individuals were excluded from participation for the following reasons: if they had used any other investigational drug or vaccine within the previous 30 days; if they were receiving immunosuppressive medication or had an immunocompromising condition; if they had previously received a meningococcal vaccine or had a history of meningococcal infection; if they had received tetanus toxoid containing vaccine within the previous month; if they had an allergy to any of the vaccine components; if they had received immunoglobulin or blood products within the 3 previous months or planned administration during the study period; if they were female and pregnant, planning to become pregnant, lactating, or unwilling to use effective contraceptive during the study; if they had a bleeding disorder; if they had a serious chronic infection or congenital defect; if they had any neurological disorder; or if they had an acute illness at the time of enrollment.
Vaccines
The MenACWY-TT investigational vaccine (GlaxoSmithKline, Belgium) contained in each 0.5 mL dose 5 µg of each polysaccharide antigen (serogroup A, C, W-135, Y) conjugated to a total of approximately 44 µg tetanus toxoid. Two lots of MenACWY-TT were included in the study: lot A had 68% and lot B had 92% O-acetylation of the MenA polysaccharide, respectively. The MenACWY-DT control vaccine (Sanofi Pasteur, Swiftwater, PA) contained in each 0.5 mL dose 4 µg of each of the 4 meningococcal serogroup polysaccharides (A, C, W-135, Y) conjugated to a total of approximately 48 µg of diphtheria toxoid.
Study Objectives
The primary study objective was to demonstrate the noninferiority of MenACWY-TT (lot A) when compared with MenACWY-DT in terms of the percentage of participants with a serum bactericidal antibody response against each serogroup 1 month postvaccination. Vaccine response was defined as a serum bactericidal titer of at least 1:8 in participants initially seronegative (titer <1:4) and as a 4-fold increase in titer in participants initially seropositive (titer ≥1:4). Secondary objectives included evaluation of the immune response by comparing geometric mean antibody titers (GMT) of the 3 vaccines; comparison of MenACWY-TT lots A and B; and evaluation of local and general solicited symptoms, unsolicited adverse events (AEs), new onsets of chronic illness, and serious AEs.
Study Procedures
After obtaining informed consent, a medical history was taken, a physical examination was performed, and 10 mL of blood was obtained by venipuncture for baseline serology. After a urine pregnancy test (in females of childbearing potential), participants were randomly allocated to receive a single dose of 0.5 mL of MenACWY-DT or 1 of the 2 lots of MenACWY-TT intramuscularly in the deltoid muscle of the nondominant arm. Randomization was performed at GlaxoSmithKline using MATEX, a program developed for use in the Statistical Analysis System software (version 9.2; SAS Institute Inc., Cary, NC). The randomization algorithm used a minimization procedure accounting for center and age strata (10 through 17 years and 18 through 25 years). The 2 vials of the 2 lots of MenACWY-TT differed in appearance and required reconstitution before injection; MenACWY-DT was supplied in a ready-to-administer liquid. Therefore, to maintain blinding, vaccine preparation and injection was performed by study personnel not otherwise involved in study evaluation procedures (observer blind). A second visit was scheduled 1 month postvaccination for repeat blood sampling for antibody determination.
Safety Assessments
Participants were monitored for 30 minutes postvaccination for any immediate AEs. Solicited injection-site reactions and systemic AEs were recorded by the participants or their parents on a diary card on the day of and for 3 days after vaccination. Solicited injection-site reactions included pain, redness, and swelling; solicited systemic AEs included fever, headache, fatigue, and gastrointestinal symptoms (abdominal pain, nausea, vomiting, and diarrhea). Injection-site redness and swelling were measured, and the greatest diameter was recorded; intensity was graded as 1 (>0 to ≤20 mm), 2 (>20 to ≤50 mm), or 3 (>50 mm). Oral temperature was recorded and graded as 1 (≥37.5○C to ≤ 38.5○C), 2 (>38.5○C to ≤ 39.5○C), or 3 (>39.5○C). Intensity of pain was graded as 1 (mild, not interfering with normal activities), 2 (moderate, painful when limb moved and interfering with normal activities), or 3 (severe, significant pain at rest and preventing normal activities). Systemic AEs were graded in relation to interference with normal activities similar to injection-site pain. Unsolicited AEs were recorded by participants or their parents on the day of and for 30 days postvaccination and graded relative to interference with normal activities. Solicited and unsolicited AEs as well as concomitant medications, new onsets of chronic illness, and serious AEs were collected by the investigator from participants or their parents at the 1-month visit. A telephone contact took place 6 months postvaccination for collection of any new onset chronic illnesses, concomitant medications, and serious AEs.
Immunogenicity Assessments
Blood samples were obtained before and 30 days after vaccination. Serum aliquots were shipped frozen to Glaxo-SmithKline Vaccine's laboratories in Rixensart, Belgium, and Laval, Canada, and were assayed by technicians blinded to vaccine allocation. Serogroup-specific, functional antimeningococcal antibodies were determined by a serum bactericidal assay using human complement (hSBA) based on the Centers for Disease Control and Prevention protocol [24]. The cutoff for the assay was a dilution of 1:4; titers were expressed as the reciprocal of the dilution resulting in 50% bacterial killing.
Statistical Considerations
The primary analysis of immunogenicity used the according-to-protocol (ATP) cohort for immunogenicity, defined as all evaluable participants (those meeting all eligibility criteria, compliance with protocol procedures, and with no elimination criteria during the study) for whom assay results were available for antibodies against at least 1 serogroup for the blood taken 1 month postvaccination (defined as 21–48 days postvaccination). The primary analysis for safety used the total vaccinated cohort (TVC), which included all vaccinated subjects for whom data were available. For each treatment group and for each antibody at each time point, the GMT and 95% confidence interval (CI) were calculated. The proportion and 95% CIs of participants with hSBA titers against prespecified cutoffs (≥1:4, ≥1:8) and the proportion and 95% CIs of participants with hSBA vaccine responses were also calculated. The primary objective of noninferiority of MenACWY-TT (lot A) compared with MenACWY-DT with respect to the serogroup A, C, W-135, Y vaccine responses were evaluated through computation of the 95% CIs for the difference in the percentage of participants with an hSBA vaccine response (MenACWY-TT lot A minus MenACWY-DT) 1 month postvaccination. Statistical noninferiority of MenACWY-TT was defined as a lower limit of the 95% CI greater than or equal to the predefined clinical limit of −10%. Exploratory immunological analyses included (1) computation of the 95% CIs of the difference in the percentage of participants with hSBA titers ≥1:4 and ≥1:8 1 month after vaccination (MenACWY-TT lot A minus MenACWY-DT, MenACWY-TT lot B minus MenACWY-DT, and MenACWY-TT lot A minus MenACWY-TT lot B) and (2) computation of the 95% CIs of the difference in the proportion of participants with hSBA vaccine response 1 month after vaccination (MenACWY-TT lot A minus MenACWY-DT and MenACWY-TT lot B minus MenACWY-DT). In the exploratory analyses, 2 vaccine groups were said to be significantly different for percentages if the 95% CI for the difference in rates did not contain the value 0. Computation of the 95% CIs of the hSBA GMT ratios (ACWY-A over ACWY-DT, ACWY-B over ACWY-DT, and ACWY-A over ACWY-B) were also performed using an analysis of covariance (ANCOVA) model on the logarithm10 transformation of the titers including the vaccine group as fixed effect and using the prevaccination (ie, the month 0 blood sampling) logarithm10 transformation of the titers and the age strata as covariates. Two groups were considered significantly different for GMTs if the 95% CI for the GMT ratio between the 2 groups did not contain the value 1. Results of the exploratory analyses should be interpreted with caution because no adjustment for multiplicity of comparisons was made.
Safety analyses included the percentage and 95% CI of participants with at least 1 injection-site AE, at least 1 systemic AE, and with any AE on the day of and 3 days postvaccination. The analysis also included the percentage and 95% CI of participants reporting individual solicited injection-site and systemic AEs (any grade, ≥grade 2, and grade 3) and relationship to vaccination, unsolicited AEs, new onset chronic illness, and serious AEs.
A sample size of 900 participants (300 per group) was calculated to provide 92% power to meet the primary objective; a sample size of 335 per group was selected to account for up to 10% of participants being excluded from the ATP cohort for immunogenicity. Statistical analyses were done using SAS software (version 9.2).
RESULTS
Demographics and Participant Disposition
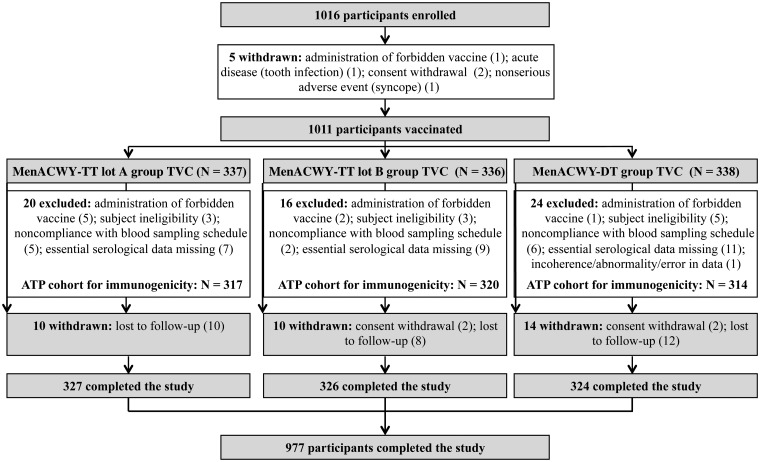
A total of 1016 participants were enrolled and 1011 participants were vaccinated (MenACWY-TT lot A 337, MenACWY-TT lot B 336, MenACWY-DT 338) (Figure 1). A total of 60 participants were excluded from the ATP for immunogenicity cohort: 20 were from MenACWY-TT lot A, 16 were from MenACWY-TT lot B, and 24 were from MenACWY-DT. Reasons for exclusion were similar amongst the 3 groups and were mostly due to participant ineligibility, noncompliance with blood sample schedule, and missing essential serological data. A total of 977 (96.6%) vaccinated participants completed the study and were equally distributed amongst the vaccine groups; the most frequent reasons for noncompletion were loss to follow-up and consent withdrawal.
Figure 1.
Participant disposition.
The 3 vaccine groups were similar for demographic characteristics (Table 1). The mean age of participants was 16.3 years (range, 10–25 years) and varied from 16.2 to 16.4 years amongst the 3 vaccine groups. A total of 58.9% of participants were between 10 and 17 years of age, and 41.1% were between 18 and 25 years of age. Females comprised 51.4% of participants, and participants ranged from 50.3% to 52.1% amongst the vaccine groups. The majority of participants (73.6%–76.0%) were of white-Caucasian/European heritage.
Table 1.
Summary of Demographic Characteristics (Total Vaccinated Cohort)
| Characteristics | Parameter or Category | MenACWY-TT lot A (N = 337) | MenACWY-TT lot B (N = 336) | MenACWY-DT (N = 338) |
|---|---|---|---|---|
| Age | Mean (SD) | 16.4 (5.16) | 16.3 (5.16) | 16.2 (4.97) |
| Gender | Female, n (%) | 175 (51.9) | 169 (50.3) | 176 (52.1) |
| Male, n (%) | 162 (48.1) | 167 (49.7) | 162 (47.9) | |
| Race | African heritage/African American, n (%) | 38 (11.3) | 29 (8.6) | 40 (11.8) |
| Asian-Central/South Asian heritage, n (%) | 17 (5.0) | 17 (5.1) | 17 (5.0) | |
| White-Caucasian/European heritage n (%) | 248 (73.6) | 249 (74.1) | 257 (76.0) | |
| Other n (%) | 34 (10.1) | 41 (12.2) | 24 (7.1) |
Abbreviations: N, total number of participants; n (%), number (percentage) of participants in a given category; SD, standard deviation.
Safety
Diary cards recording AEs were returned by most participants (97.6% of MenACWY-TT lot A, 97.9% of MenACWY-TT lot B, and 96.2% and 96.4% of MenACWY-DT for solicited injection-site and systemic AEs, respectively). At least 1 symptom was reported during the 4-day safety follow-up period in 70.0% of MenACWY-TT lot A recipients, 66.1% of MenACWY-TT lot B recipients, and 70.1% of MenACWY-DT recipients. Injection-site reactions were reported by 58.8%, 56.3%, and 59.5% of recipients, respectively, and systemic AEs were reported by 44.8%, 39.3%, and 42.3% of recipients, respectively. Injection-site pain was the most frequently reported solicited AE, and headache and fatigue were the most common systemic AEs reported (Table 2). Grade 3 (severe) injection-site and systemic AEs were uncommon. Rates of injection-site and systemic AEs and grade 3 AEs were similar amongst the 3 vaccine groups.
Table 2.
Percentage of Participants Experiencing Solicited Local and General Symptoms in the MenACWY-TT and MenACWY-DT Groups During 4-Day Period After Vaccination (Total Vaccinated Cohort)
| Symptom | Type | MenACWY-TT lot A (N = 329) |
MenACWY-TT lot B (N = 329) |
MenACWY-DT (N = 325) |
|||
|---|---|---|---|---|---|---|---|
| n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | ||
| Local | |||||||
| Pain | All | 169 | 51.4 (45.8, 56.9) | 167 | 50.8 (45.2, 56.3) | 180 | 55.4 (49.8, 60.9) |
| Grade 3 | 8 | 2.4 (1.1, 4.7) | 5 | 1.5 (0.5, 3.5) | 2 | 0.6 (0.1, 2.2) | |
| Redness (mm) | All | 85 | 25.8 (21.2, 30.9) | 60 | 18.2 (14.2, 22.8) | 66 | 20.3 (16.1, 25.1) |
| Grade 3 | 3 | 0.9 (0.2, 2.6) | 2 | 0.6 (0.1, 2.2) | 6 | 1.8 (0.7, 4.0) | |
| Swelling (mm) | All | 63 | 19.1 (15.0, 23.8) | 40 | 12.2 (8.8, 16.2) | 44 | 13.5 (10.0, 17.7) |
| Grade 3 | 3 | 0.9 (0.2, 2.6) | 3 | 0.9 (0.2, 2.6) | 3 | 0.9 (0.2, 2.7) | |
| General | |||||||
| Fatigue | All | 96 | 29.2 (24.3, 34.4) | 94 | 28.6 (23.8, 33.8) | 89 | 27.3 (22.5, 32.5) |
| Grade 3 | 9 | 2.7 (1.3, 5.1) | 7 | 2.1 (0.9, 4.3) | 5 | 1.5 (0.5, 3.5) | |
| Gastrointestinala | All | 43 | 13.1 (9.6, 17.2) | 43 | 13.1 (9.6, 17.2) | 44 | 13.5 (10.0, 17.7) |
| Grade 3 | 4 | 1.2 (0.3, 3.1) | 3 | 0.9 (0.2, 2.6) | 4 | 1.2 (0.3, 3.1) | |
| Headache | All | 86 | 26.1 (21.5, 31.2) | 87 | 26.4 (21.8, 31.6) | 83 | 25.5 (20.8, 30.6) |
| Grade 3 | 5 | 1.5 (0.5, 3.5) | 2 | 0.6 (0.1, 2.2) | 6 | 1.8 (0.7, 4.0) | |
| Fever | All | 17 | 5.2 (3.0, 8.1) | 14 | 4.3 (2.3, 7.0) | 16 | 4.9 (2.8, 7.8) |
| Grade 3b | 1 | 0.3 (0.0, 1.7) | 0 | 0 (0.0, 1.1) | 0 | 0 (0.0, 1.1) | |
Abbreviations: CI, confidence interval; N, total number of participants, with the documented dose; n/%, number/percentage of participants reporting the symptom at least once.
aGastrointestinal symptoms included nausea, vomiting, diarrhea, and/or abdominal pain.
bTemperature >39.5°C.
At the 6-month follow-up, at least 1 new onset chronic illness was reported by 3 participants in the MenACWY-TT lot A group, including hypersensitivity, insulin resistance, asthma, and bronchial hyperreactivity; none were reported in the other 2 groups. Serious AEs were reported by 1 participant in the MenACWY-TT lot A group (asthma), 5 participants in the MenACWY-TT lot B group (8 serious AEs: tooth infection; appendicitis; asthma, influenza and pneumonia; and pneumonia and hypoxia), and 2 participants in the MenACWY-DT group (jaw fracture and postprocedural hematoma); none of the serious AEs were considered by the investigators to be vaccine-related.
Immunogenicity
At baseline, 79 (25.5%) of 310 participants in the MenACWY-TT lot A group, 84 (27.2%) of 309 in the MenACWY-TT lot B group, and 89 (29.1%) of 306 participants in the MenACWY-DT group were seropositive for MenA antibody. For MenC antibody, 175 (60.8%) of 288 in the MenACWY-TT lot A group, 188 (65.7%) of 286 in the MenACWY lot B group, and 197 (68.2%) of 289 in the MenACWY-DT group were seropositive. For MenW-135 antibody, 99 (33.8%) of 293 in the MenACWY-TT lot A group, 98 (33.8%) of 290 in the MenACWY-TT lot B group, and 102 (34.1%) of 299 in the MenACWY-DT group were seropositive. For MenY antibody, 215 (72.6%) of 296 in the MenACWY-TT lot A group, 224 (73.2%) of 306 in the MenACWY-TT lot B group, and 234 (77.0%) of 304 in the MenACWY-DT group were seropositive. The proportion of participants in each vaccine group with a vaccine response against the 4 serogroups is depicted in Table 3. Vaccine response rates were consistently higher in participants who were initially seronegative at baseline (data not shown). The noninferiority of MenACWY-TT (lot A) compared with MenACWY-DT was demonstrated in terms of the percentage of participants with a vaccine response as measured by hSBA against serogroups A, C, W-135, and Y 1 month after vaccination. The difference in vaccine response rate (MenACWY-TT lot A minus MenACWY-DT) was 6.01% (95% CI −1.45 to 13.44) for MenA, 0.95% (−6.10 to 8.00) for MenC, 6.95% (−0.76 to 14.59) for MenW-135, and 12.21% (4.17–20.10) for MenY, all above the noninferiority threshold for the lower limit of the 95% CI of −10%. In the exploratory analyses, a statistically significantly higher vaccine response rate was observed for MenACWY-TT lot A compared with MenACWY-DT for hSBA Men Y and for MenACWY-TT lot B compared with MenACWY-DT for hSBA MenW-135 and MenY. No statistically significant differences were detected for vaccine response between any of the serogroups between MenACWY-TT lots A and B.
Table 3.
Geometric Mean Antibody Titers and Percentage of Participants With a Vaccine Response at 1 Month After Vaccination in the MenACWY-TT and MenACWY-DT Groups (ATP Cohort for Immunogenicity)
| Antibody | GMT (95% CI) |
Adjusted GMT Group Ratio MenACWY-TT lot A or B/MenACWY-DT | Adjusted GMT Group Ratio MenACWY-TT lot A/MenACWY-TT lot B | Vaccine Response |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Group | N | Pre | N | Post | (95% CI) | (95% CI) | N | %a (95% CI) | |
| MenA | MenACWY-TT lot A | 310 | 3.6 (3.1, 4.0) | 315 | 54.2 (43.5, 67.4) | 1.34 (0.97, 1.84) | 1.08 (0.79, 1.47) | 310 | 70.3 (64.9, 75.4) |
| MenACWY-TT lot B | 309 | 3.6 (3.2, 4.1) | 309 | 49.6 (39.6, 62.1) | 1.24 (0.90, 1.73) | – | 300 | 71.3 (65.9, 76.4) | |
| MenACWY-DT | 306 | 3.6 (3.2, 4.1) | 305 | 41.3 (32.3, 52.9) | – | – | 297 | 64.3 (58.6, 69.8) | |
| MenC | MenACWY-TT lot A | 288 | 15.6 (12.3, 19.9) | 307 | 687.1 (510.5, 924.9) | 1.35 (0.92, 1.99) | 0.92 (0.62, 1.38) | 281 | 77.2 (71.9, 82.0) |
| MenACWY-TT lot B | 286 | 16.0 (12.8, 20.0) | 304 | 755.8 (557.3, 1025.0) | 1.47 (1.00, 2.17) | – | 274 | 82.5 (77.5, 86.8) | |
| MenACWY-DT | 289 | 18.0 (14.4, 22.6) | 296 | 543.3 (411.2, 718.0) | – | – | 274 | 76.3 (70.8, 81.2) | |
| MenW-135 | MenACWY-TT lot A | 293 | 7.7 (6.1, 9.7) | 298 | 174.5 (138.6, 219.6) | 1.60 (1.15, 2.23)b | 1.04 (0.76, 1.43) | 279 | 71.0 (65.3, 76.2) |
| MenACWY-TT lot B | 290 | 7.6 (6.0, 9.6) | 292 | 161.6 (128.3, 203.5) | 1.55 (1.11, 2.15) | – | 270 | 72.6 (66.9, 77.8) | |
| MenACWY-DT | 299 | 7.4 (5.9, 9.2) | 297 | 101.7 (77.9, 132.7) | – | – | 289 | 64.0 (58.2, 69.6) | |
| MenY | MenACWY-TT lot A | 296 | 45.7 (35.9, 58.2) | 313 | 349.1 (298.1, 408.8) | 1.54 (1.21, 1.97) | 0.91 (0.73, 1.14) | 293 | 51.2 (45.3, 57.1) |
| MenACWY-TT lot B | 306 | 49.8 (39.1, 63.4) | 307 | 387.4 (329.7, 455.1) | 1.66 (1.30, 2.13) | – | 294 | 51.0 (45.2, 56.9) | |
| MenACWY-DT | 304 | 55.3 (43.7, 69.9) | 305 | 253.8 (204.9, 314.5) | – | – | 295 | 39.0 (33.4, 44.8) | |
Abbreviations: Adjusted GMT, geometric mean antibody titer adjusted for age strata and baseline titer; ATP, according to protocol; CI, confidence interval; GMT, geometric mean antibody titer (reciprocal dilution) calculated on all participants; N, number of subjects with results available; post, postvaccination at month 1; pre, prevaccination at month 0.
aPercentage of participants with a vaccine response.
bBolded text indicates statistically significant group difference in GMT based on exploratory analysis.
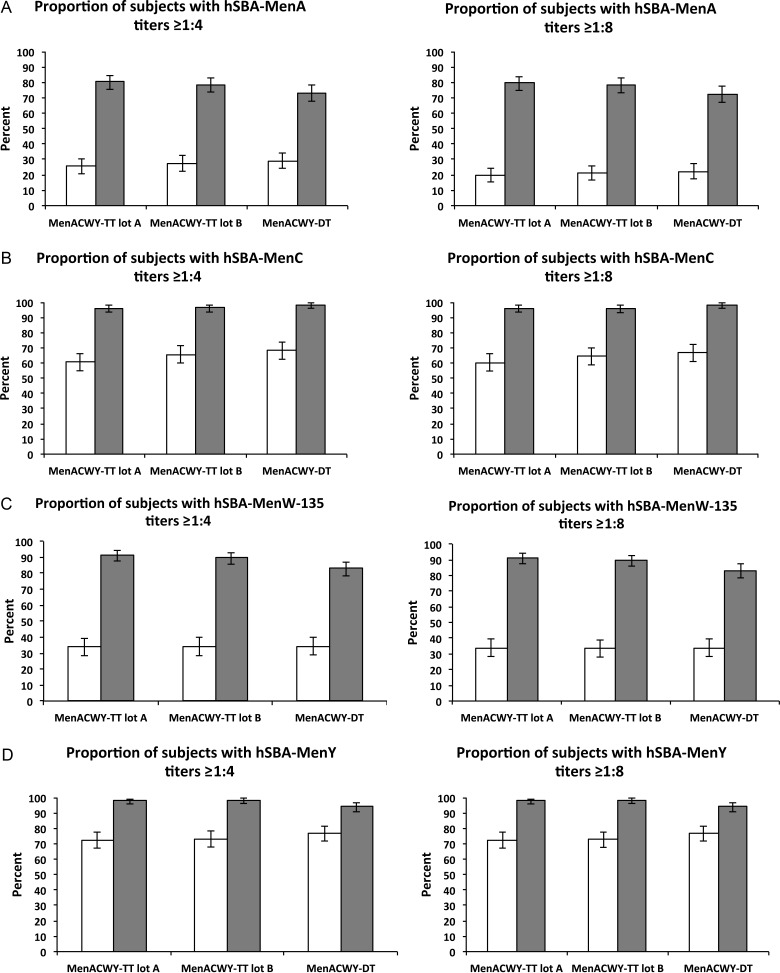
Prevaccination, GMTs against the 4 serogroups were similar amongst the 3 vaccine groups (Table 3). Postvaccination, all groups demonstrated increased GMTs against all 4 serogroups. Differences in GMTs between groups were explored using GMT ratios; GMTs for serogroups W-135 and Y were statistically higher in recipients of either MenACWY-TT lot A or MenACWY-TT lot B compared with MenACWY-DT; no differences were observed between MenACWY-TT (lots A and B). The proportion of participants postvaccination with hSBA titers ≥1:4 for the 4 serogroups ranged between 73.1% and 80.3% for serogroup A, between 96.1% and 98.3% for serogroup C, between 83.2% and 91.3% for serogroup W-135, and between 94.1% and 98.4% for serogroup Y (Figure 2). The percentage of participants with antibody titers ≥1:4 was significantly higher at day 28 for recipients of MenACWY-TT lot A for serogroups A, W-135, and Y compared with MenACWY-DT, and statistically higher for recipients of MenACWY-TT lot B for serogroups W-135 and Y compared with MenACWY-DT. There were no differences between MenACWY-TT lots A and B. A similar pattern was observed for the proportion of participants with postvaccination titers ≥1:8; most participants who achieved protective titers of ≥1:4 also exceeded the ≥1:8 threshold (Figure 2).
Figure 2.
Percentage of participants with hSBA titers equal to or above the cutoff values of 1:4 (left) and 1:8 (right) against (A) MenA, (B) MenC, (C) MenW-135, (D) MenY prevaccination (open bars), and postvaccination (closed bars) in the according-to-protocol cohort for immunogenicity. Error bars represent the 95% confidence intervals.
DISCUSSION
In this study, MenACWY-TT met the primary immunogenicity noninferiority vaccine response criteria relative to a marketed MenACWY-DT vaccine for all 4 serogroups in adolescents and young adults. In the secondary immunogenicity analyses, both lots of MenACWY-TT elicited significantly higher GMTs against serogroups W-135 and Y and proportions of participants achieving titers ≥1:4 against serogroups W-135 and Y, as well as the proportion achieving titers ≥1:4 for lot A against serogroup A. Taken in aggregate, the data suggest that the immunogenicity of both lots of MenACWY-TT was at least as high as that of the licensed comparator vaccine for all 4 serogroups.
Injection-site and systemic AEs were similar between recipients of both lots of MenACWY-TT and MenACWY-DT. Pain was the most common injection-site reaction, and headache and fatigue were the most commonly reported systemic AEs. Although new onset chronic illness was reported only by recipients of MenACWY-TT, the events did not represent a specific disease or syndrome; all 3 participants were in lot A group, likely representing chance occurrence. Serious AEs were reported by 6 MenACWY-TT participants (1 in lot A and 5 in lot B, likely representing chance clustering) and 2 MenACWY-DT recipients, reflecting the 2:1 MenACWY-TT/MenACWY-DT allocation.
The immunogenicity results of this study support the findings of a previous study of 784 adolescents and young adults 11–25 years of age who received a single dose of MenACWY-TT or MenACWY-DT where similarity of the MenACWY-TT vaccine was demonstrated in exploratory analyses as well as significantly higher GMTs and proportions of participants achieving hSBA titers ≥1:4 for some or all serogroups [20]. The current study also demonstrated that the 2 lots of MenACWY-TT with differing levels of O-acetylation did not differ in either reactogenicity or immunogenicity. The MenA capsular polysaccharide is approximately 70%–95% O-acetylated at carbon 3 [23, 25, 26]. The 2 lots of MenACWY-TT vaccine spanned the range of O-acetylation and performed similarly in this study. O-acetylation is an important factor in the immunogenicity of MenA polysaccharide conjugates; deacetylation during the conjugation process has been previously reported to markedly affect the immunogenicity of MenA vaccines in bactericidal assays [23]; however, this was not confirmed in our study.
At the time of this clinical trial, 2 other quadrivalent meningococcal conjugate vaccines were marketed worldwide. Menactra (Sanofi Pasteur, Swiftwater, PA), which uses diphtheria toxoid as the carrier protein, was the first quadrivalent meningococcal conjugate vaccine to market, and it was used as the comparator vaccine in this study. Menveo (Novartis Vaccines and Diagnostics, Cambridge, MA), which uses CRM197 as the carrier protein, was second to market, and it demonstrated noninferiority to Menactra in a prelicensure clinical trial conducted in adolescents [27]. Similar to this study with the novel MenACWY-TT vaccine, Menveo showed increased GMTs compared with Menactra [27]. MenACWY-TT has also been shown to be noninferior to quadrivalent meningococcal polysaccharide vaccine in children aged 2 to 10 years [21], Asian adolescents [18], and European adolescents and young adults [15]. Four different formulations of the novel MenACWY-TT vaccine compared with age-appropriate, marketed meningococcal vaccines (either MenC conjugate vaccine or quadrivalent meningococcal conjugate vaccine) were studied in children 1–5 years of age [16], and noninferiority of MenACWY-TT compared with MenC was demonstrated in children 12–23 months of age [22]. There was no detrimental effect on reactogenicity or immunogenicity when MenACWY-TT was coadministered with a combined diphtheria, tetanus, acellular pertussis, inactivated poliovirus, Haemophilus influenzae type b conjugate, hepatitis B vaccine in toddlers [17], with combined measles-mumps-rubella-varicella vaccine in toddlers [22], or combined hepatitis A-hepatitis B vaccine in adolescents [19]. In this study, MenACWY‐TT vaccine was well tolerated and elicited an immune response that was noninferior to that of a marketed MenACWY‐DT vaccine. Taken collectively, these studies provide the data needed to support the use of MenACWY-TT in various national meningococcal vaccination programs. In the United States, quadrivalent meningococcal conjugate vaccines are licensed and recommended for all adolescents, as well as for children 9 months to 2 years (2-dose regimen) of age and individuals 2–55 years of age (single dose) with conditions that put them at increased risk for invasive meningococcal disease [28–32]. In Canada, some provinces recommend an adolescent booster dose of quadrivalent meningococcal conjugate vaccines after infant MenC conjugate vaccination [33]. Most European countries have implemented MenC conjugate vaccination programs because of the predominance of serogroup C disease [34]. MenACWY-TT has now received market authorization in the European Union for administration as a single dose to individuals 12 months of age or older, expanding the options for invasive meningococcal disease control.
Acknowledgments
We thank the individuals and their parents or legal guardians who participated in the study, and we thank all of the other investigators involved in conducting the study (T. Araki, D. Connor, B. Lasko, D. Shu, A. McIntosh, W. Andrews, R. Broker, W. Ellison, R. Hines, I. Rarick, J. Borders, B. Fox, N. Segall, and L. Wadsworth). We also thank the nurses and research assistants at all enrollment sites for their careful attention to detail. Finally, we thank F. Del Buono (GlaxoSmithKline Vaccines) for protocol development, GlaxoSmithKline Vaccines study manager A. Lee, and B. van Heertum (XPE Pharma & Science on behalf of GlaxoSmithKline Vaccines) for manuscript coordination.
Financial support. This work was supported by GlaxoSmithKline Biologicals SA.
Potential conflicts of interest S. A. H., J. M. L., and S. A. M. have served on ad hoc advisory boards for the GlaxoSmithKline group of companies and other vaccine manufacturers and governments. J. B. D. received compensation for lectures from the GlaxoSmithKline group of companies. C. I. B., L. R. F., Y. B., V. B., and J. M. M. are employees of the GlaxoSmithKline group of companies. C. I. B., L. R. F., Y. B., and J. M. M. declare stock ownership in the GlaxoSmithKline group of companies.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Harrison LH, Trotter CL, Ramsay ME. Global epidemiology of meningococcal disease. Vaccine. 2009;275:B51–63. doi: 10.1016/j.vaccine.2009.04.063. [DOI] [PubMed] [Google Scholar]
- 2.Rosenstein NE, Perkins BA, Stephens DS, et al. Meningococcal disease. N Engl J Med. 2001;344:1378–88. doi: 10.1056/NEJM200105033441807. [DOI] [PubMed] [Google Scholar]
- 3.Harrison LH. Prospects for vaccine prevention of meningococcal infection. Clin Microbiol Rev. 2006;19:142–64. doi: 10.1128/CMR.19.1.142-164.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halperin SA, Bettinger JA, Greenwood B, et al. The changing and dynamic epidemiology of meningococcal disease. Vaccine. 2012;30(Suppl 2) doi: 10.1016/j.vaccine.2011.12.032. B26–36. [DOI] [PubMed] [Google Scholar]
- 5.NNDSS Annual Report Writing Group. Australia's notifiable disease status, 2008: Annual report of the National Notifiable Diseases Surveillance System. Commun Dis Intell. 2010;34:214–6. [PubMed] [Google Scholar]
- 6.Trotter C, Gay N, Edmunds W. Dynamic models of meningococcal carriage and the impact of serogroup C conjugate vaccination. Am J Epidemiol. 2005;162:89–100. doi: 10.1093/aje/kwi160. [DOI] [PubMed] [Google Scholar]
- 7.de Greeff SC, de Melker HE, Spanjaard L, et al. Protection from routine vaccination at the age of 14 months with meningococcal serogroup C conjugate vaccine in the Netherlands. Pediatr Infect Dis J. 2006;25:79–80. doi: 10.1097/01.inf.0000195594.41449.c6. [DOI] [PubMed] [Google Scholar]
- 8.De Wals P, Deceuninck G, Lefebvre B, et al. Effectiveness of serogroup C meningococcal conjugate vaccine. A 7-year follow-up in Quebec, Canada. Pediatr Infect Dis J. 2011;30:566–9. doi: 10.1097/INF.0b013e31820e8638. [DOI] [PubMed] [Google Scholar]
- 9.Bettinger JA, Scheifele DW, Le Saux N, et al. The impact of childhood meningococcal serogroup C conjugate vaccine programs in Canada. Pediatr Infect Dis J. 2009;28:220–4. doi: 10.1097/INF.0b013e31819040e7. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC) Updated recommendations for use of meningococcal conjugate vaccines—Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2011;60:72–76. [PubMed] [Google Scholar]
- 11.Canadian Immunization Committee and Public Health Agency of Canada. Advice for consideration of quadrivalent (A, C, Y, W135) meningococcal conjugate vaccine, for use by provinces and territories. Can Commun Dis Rep. 2010;36(Suppl 2):1–36. doi: 10.14745/ccdr.v36i00as2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Granoff DM. Review of meningococcal group B vaccines. Clin Infect Dis. 2010;50(Suppl 2) doi: 10.1086/648966. S54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serruto D, Bottomley MJ, Ram S, et al. The new multicomponent vaccine against meningococcal serogroup B, 4CMenB: immunological, functional and structural characterization of the antigens. Vaccine. 2012;30(Suppl 2) doi: 10.1016/j.vaccine.2012.01.033. B87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richmond PC, Marshall HS, Nissen MD, et al. Safety, immunogenicity, and tolerability of meningococcal serogroup B bivalent recombinant lipoprotein 2086 vaccine in healthy adolescents: a randomised, single-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis. 2012;12:597–607. doi: 10.1016/S1473-3099(12)70087-7. [DOI] [PubMed] [Google Scholar]
- 15.Ostergaard L, Lebacq E, Poolman J, et al. Immunogenicity, reactogenicity and persistence of meningococcal A, C, W-135 and Y-tetanus toxoid candidate conjugate (MenACWY-TT) vaccine formulations in adolescents aged 15–25 years. Vaccine. 2009;27:161–8. doi: 10.1016/j.vaccine.2008.08.075. [DOI] [PubMed] [Google Scholar]
- 16.Knuf M, Kieninger-Baum D, Habermehl P, et al. A dose-range study assessing immunogenicity and safety of one dose of a new candidate meningococcal serogroups A, C, W-135, Y tetanus toxoid conjugate (MenACWY-TT) vaccine administered in the second year of life and in young children. Vaccine. 2010;28:744–53. doi: 10.1016/j.vaccine.2009.10.064. [DOI] [PubMed] [Google Scholar]
- 17.Knuf M, Pantazi-Chatzikonstantinou A, Pfletschinger U, et al. An investigational tetravalent meningococcal serogroups A, C, W-135 and Y-tetanus toxoid conjugate vaccine co-administered with Infanrix™ hexa is immunogenic, with an acceptable safety profile in 12–23-month-old children. Vaccine. 2011;29:4264–73. doi: 10.1016/j.vaccine.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 18.Bermal N, Huang LM, Dubey AP, et al. Safety and immunogenicity of a tetravalent meningococcal serogroups A, C, W-135 and Y conjugate vaccine in adolescents and adults. Hum Vaccin. 2011;7:239–47. doi: 10.4161/hv.7.2.14068. [DOI] [PubMed] [Google Scholar]
- 19.Østergaard L, Silfverdal SA, Berglund J, et al. A tetravalent meningococcal serogroups A, C, W-135, and Y tetanus toxoid conjugate vaccine is immunogenic and well-tolerated when co-administered with Twinrix® in subjects aged 11–17 years: An open, randomised, controlled trial. Vaccine. 2012;30:774–83. doi: 10.1016/j.vaccine.2011.11.051. [DOI] [PubMed] [Google Scholar]
- 20.Baxter R, Baine Y, Ensor K, et al. Immunogenicity and safety of an investigational quadrivalent meningococcal ACWY tetanus toxoid conjugate vaccine in healthy adolescents and young adults 10 to 25 years of age. Pediatr Infect Dis J. 2011;30 doi: 10.1097/INF.0b013e3182054ab9. e41–8. [DOI] [PubMed] [Google Scholar]
- 21.Memish ZA, Dbaibo G, Montellano M, et al. Immunogenicity of a single dose of tetravalent meningococcal serogroups A, C, W-135, and Y conjugate vaccine administered to 2- to 10-year-olds is noninferior to a licensed-ACWY polysaccharide vaccine with an acceptable safety profile. Pediatr Infect Dis J. 2011;30 doi: 10.1097/INF.0b013e31820e6e02. e56–62. [DOI] [PubMed] [Google Scholar]
- 22.Vesikari T, Karvonen A, Bianco V, et al. Tetravalent meningococcal serogroups A, C, W-135 and Y conjugate vaccine is well tolerated and immunogenic when co-administered with measles-mumps-rubella-varicella vaccine during the second year of life: an open, randomized controlled trial. Vaccine. 2011;29:4274–84. doi: 10.1016/j.vaccine.2011.03.043. [DOI] [PubMed] [Google Scholar]
- 23.Berry DS, Lynn F, Lee CH, et al. Effect of O acetylation of Neisseria meningitidis serogroup A capsular polysaccharide on development of functional immune responses. Infect Immun. 2002;70:3707–13. doi: 10.1128/IAI.70.7.3707-3713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maslanka SE, Gheesling LL, Libutti DE, et al. Standardization and a multilaboratory comparison of Neisseria meningitidis serogroup A and C serum bactericidal assays. The Multilaboratory Study Group. Clin Diagn Lab Immunol. 1997;4:156–67. doi: 10.1128/cdli.4.2.156-167.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jennings H, Bhattacharjee A, Bundle D, et al. Structure of the capsular polysaccharide of Neisseria meningitidis as determined by 13C-nuclear magnetic resonance spectroscopy. J Infect Dis. 1977;136 doi: 10.1093/infdis/136.supplement.s78. S78–83. [DOI] [PubMed] [Google Scholar]
- 26.Lemercinier X, Jones C. Full 1H NMR assignment and detailed O-acetylation patterns of capsular polysaccharides used in vaccine production. Carbohydr Res. 1996;296:83–96. doi: 10.1016/s0008-6215(96)00253-4. [DOI] [PubMed] [Google Scholar]
- 27.Jackson LA, Baxter R, Reisinger K, et al. Phase III comparison of an investigational quadrivalent meningococcal conjugate vaccine with the licensed meningococcal ACWY conjugate vaccine in adolescents. Clin Infect Dis. 2009;49 doi: 10.1086/599117. e1–10. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention (CDC) Revised recommendations of the Advisory Committee on Immunization Practices to vaccinate all persons aged 11–18 years with meningococcal conjugate vaccine. MMWR Morb Mortal Wkly Rep. 2007;56:794–5. [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention (CDC) Report from the Advisory Committee on Immunization Practices (ACIP): Decision not to recommend routine immunization of all children aged 2–10 years with quadrivalent meningococcal conjugate vaccine (MCV4) MMWR Morb Mortal Wkly Rep. 2008;57:462–5. [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention (CDC) Notice to readers: recommendations from the Advisory Committee on Immunization Practices (ACIP) for use of quadrivalent meningococcal conjugate vaccine (MCV4) in children aged 2–10 years at increased risk for invasive meninogocccal disease. MMWR Morb Mortal Wkly Rep. 2007;56:1265–6. [Google Scholar]
- 31.Centers for Disease Control and Prevention (CDC) General recommendations on immunization. Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2011;60:1–61. [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention (CDC) Recommendation of the Advisory Committee on Immunization Practices (ACIP) for use of quadrivalent meningococcal conjugate vaccine (MenACWY-D) among children aged 9 through 23 months at increased risk for invasive meningococcal disease. MMWR Morb Mortal Wkly Rep. 2011;60:1391–2. [PubMed] [Google Scholar]
- 33.Public Health Agency of Canada. Publicly funded immunization programs in Canada—Routine schedule for infants and children including special programs and catch-up programs. Available at: http://www.phac-aspc.gc.ca/im/ptimprog-progimpt/table-1-eng.php . Accessed 6 October 2012. [Google Scholar]
- 34.Trotter CL, Ramsay ME. Vaccination against meningococcal disease in Europe: review and recommendations for the use of conjugate vaccines. FEMS Microbiol Rev. 2007;31:101–7. doi: 10.1111/j.1574-6976.2006.00053.x. [DOI] [PubMed] [Google Scholar]