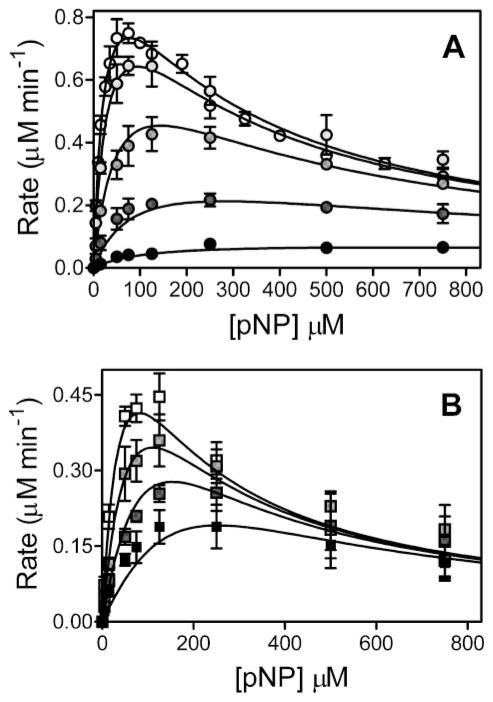
FIGURE 2.
Steady-state oxidation of pNP by CYP2E1 in the presence of 4MP (A) or IND (B). For reactions, 25 nM CYP2E1, 100 nM CPR-K56Q, and 50 nM cytochrome b5 were reconstituted in 50 mM potassium phosphate, pH 7.4, 20 μM DLPC, pNP (varied from 5 to 750 μM), 2 units μl−1 catalase, 0.04 μg μl−1 superoxide dismutase, and an NADPH-regenerating system (2 microunits μl−1 glucose-6-phosphate dehydrogenase, 10 mM glucose 6-phosphate, 2 mM MgCl2, 500 μM NADP+) at 37 °C. To determine initial velocities, product p-nitrocatechol was quantitated as a function of time by HPLC as described (11). The reported values reflect the average from 2 to 4 experiments, including the mean ± S.D. A, for 4MP studies, 0, 1, 5, 25, and 125 μM inhibitor was added to reactions. Data were fit to model 2a in Scheme 2. B, IND studies were carried out in the presence of 0.1, 0.3, and 1.0 μM inhibitor at a final concentration of 0.25% methanol. Final data were fit to the single-site competition mechanism (model 1) shown in Scheme 2.