Figure 1.
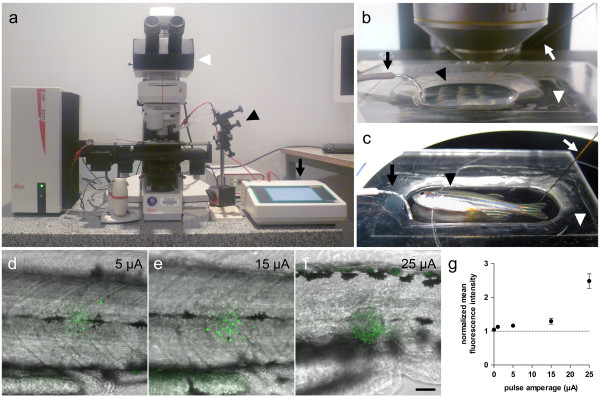
Electroablation setup and dependence of tissue damage on amperage. (a-c) Experimental setup for electroablation in zebrafish larvae (b) and adults (c). (a) Current pulses are generated by a precision current source (black arrow), which is connected to the sample by the microelectrode and a ground wire. The microelectrode is held by a micromanipulator (black arrowhead) and zebrafish are visualized under a fluorescence microscope (white arrowhead). (b) Close-up view of experimental setup for electroablation in larvae. The microelectrode (white arrow) enters the agarose through one side and a ground wire is connected to the other side (black arrow). Zebrafish larvae are mounted in a drop of agarose dissolved in E3 (black arrowhead) in the central depression of the acrylic plate (white arrowhead). (c) Adult zebrafish (black arrowhead) are positioned in an acrylic plate (white arrowhead) with a larger depression and with a small amount of E3. The microelectrode (white arrow) touches the caudal fin and the ground wire is immersed in the E3 medium (black arrow). (d-f) Merge of acridine orange (green) and bright field images showing progressive expansion of acridine orange stain as amperage increases. Pulses of different amperages (5 – 25 μA) were applied for 1 second, and acridine orange stain was performed 2 hpi. (g) Quantification of acridine orange fluorescence shows a direct relationship with applied amperage. Average fluorescence intensity was measured within a 50 μm radius surrounding the electroablation site. Data was normalized for each larva by the average fluorescence intensity measured in an adjacent uninjured area. Values are presented as a normalized average ± SEM from 15–21 larvae per condition, from three independent experiments. Scale bar, 50 μm.
