Abstract
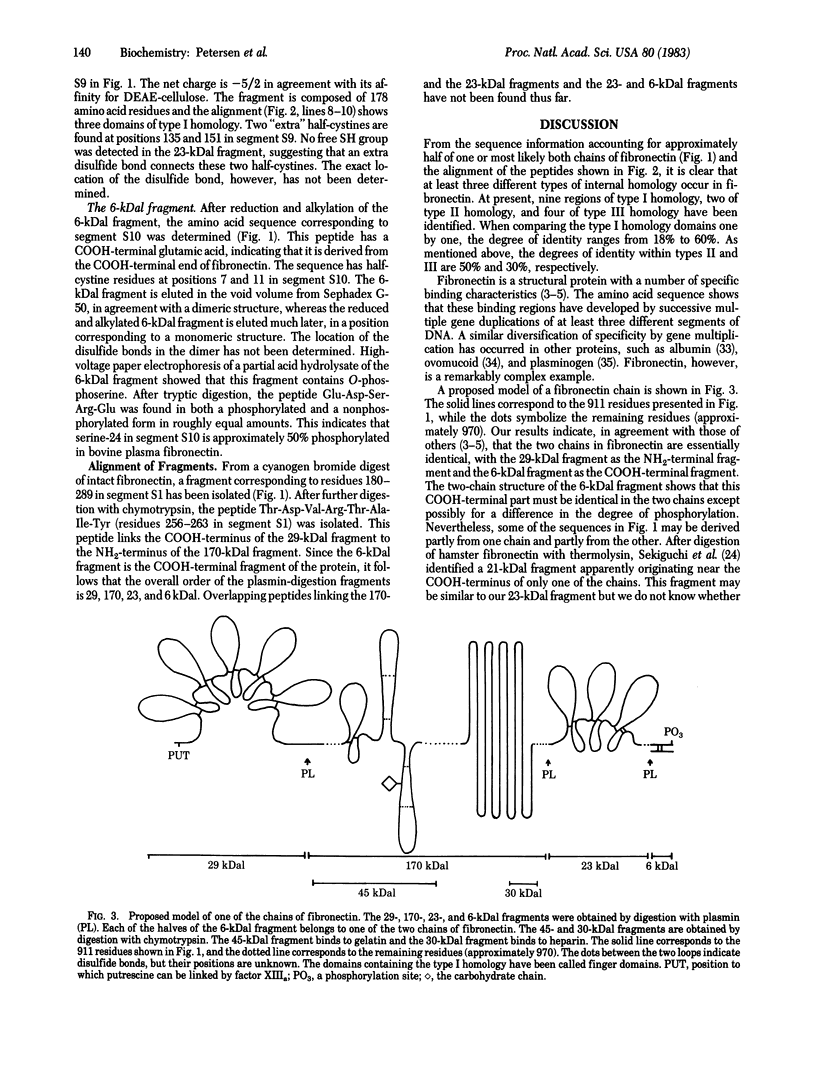
Approximately one-half of the amino acid sequence (911 amino acid residues out of 1,880 expected) for bovine plasma fibronectin (cold-insoluble globulin) has been determined. Three types of internal homology were identified, showing that a number of partial gene duplications (multiplications) have occurred during the evolution of this protein. Digestion of fibronectin with plasmin results in major fragments with molecular masses of 29, 170, 23, and 6 kilodaltons (kDal). The NH2-terminal 29-kDal fragment consists of 259 residues ordered as five mutually homologous domains (type I homology) with two disulfide bonds in each domain. The 170-kDal fragment shows two to three bands after NaDodSO4 gel electrophoresis, indicating heterogeneity. This fragment contains the gelatin binding site and the strong heparin binding site present in fibronectin. Digestion of the 170-kDal fragment with chymotrypsin liberates a 45-kDal fragment that also binds to gelatin. This fragment contains at least one domain of type I homology and two domains of type II homology. Further digestion of the 170-kDal fragment with chymotrypsin results in the formation of a 30-kDal fragment that retains the heparin binding activity. This fragment contains sequences constituting type III homology. The 23-kDal fragment consists of 178 residues having three domains of type I homology. The 6-kDal fragment consists of two identical peptides of 26 residues, and these two peptides are linked to each other by two disulfide bonds that form the interchain bridges. Another one of the peptides for which the sequence was determined links the COOH-terminus of the 29-kDal fragment to the NH2-terminus of the 170-kDal fragment. This and the fact that the COOH-terminal residue of the 6-kDal fragment is a glutamic acid residue order the four plasmin-digestion fragments as 29-, 170-, 23-, and 6-kDal in the intact fibronectin molecule.
Keywords: plasmin digestion, gelatin and heparin binding, interchain disulfide bridge, phosphorylation, gene multiplication
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali I. U., Hunter T. Structural comparison of fibronectins from normal and transformed cells. J Biol Chem. 1981 Jul 25;256(14):7671–7677. [PubMed] [Google Scholar]
- Balian G., Click E. M., Crouch E., Davidson J. M., Bornstein P. Isolation of a collagen-binding fragment from fibronectin and cold-insoluble globulin. J Biol Chem. 1979 Mar 10;254(5):1429–1432. [PubMed] [Google Scholar]
- Chen A. B., Amrani D. L., Mosesson M. W. Heterogeneity of the cold-insoluble globulin of human plasma (CIg), a circulating cell surface protein. Biochim Biophys Acta. 1977 Aug 23;493(2):310–322. doi: 10.1016/0005-2795(77)90187-8. [DOI] [PubMed] [Google Scholar]
- De Petro G., Barlati S., Vartio T., Vaheri A. Transformation-enhancing activity of gelatin-binding fragments of fibronectin. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4965–4969. doi: 10.1073/pnas.78.8.4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmerling M. R., Johnson C. D., Mosher D. F., Lipton B. H., Lilien J. E. Cross-linking and binding of fibronectin with asymmetric acetylcholinesterase. Biochemistry. 1981 May 26;20(11):3242–3247. doi: 10.1021/bi00514a040. [DOI] [PubMed] [Google Scholar]
- Furie M. B., Rifkin D. B. Proteolytically derived fragments of human plasma fibronectin and their localization within the intact molecule. J Biol Chem. 1980 Apr 10;255(7):3134–3140. [PubMed] [Google Scholar]
- Gold L. I., Garcia-Pardo A., Frangione B., Franklin E. C., Pearlstein E. Subtilisin and cyanogen bromide cleavage products of fibronectin that retain gelatin-binding activity. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4803–4807. doi: 10.1073/pnas.76.10.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn L. H., Yamada K. M. Isolation and biological characterization of active fragments of the adhesive glycoprotein fibronectin. Cell. 1979 Dec;18(4):1043–1051. doi: 10.1016/0092-8674(79)90217-4. [DOI] [PubMed] [Google Scholar]
- Jones M. D., Petersen T. E., Nielsen K. M., Magnusson S., Sottrup-Jensen L., Gausing K., Clark B. F. The complete amino-acid sequence of elongation factor Tu from Escherichia coli. Eur J Biochem. 1980 Jul;108(2):507–526. doi: 10.1111/j.1432-1033.1980.tb04748.x. [DOI] [PubMed] [Google Scholar]
- Keski-Oja J., Sen A., Todaro G. J. Direct association of fibronectin and actin molecules in vitro. J Cell Biol. 1980 Jun;85(3):527–533. doi: 10.1083/jcb.85.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman H. K., Martin G. R., Fishman P. H. Ganglioside inhibition of fibronectin-mediated cell adhesion to collagen. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3367–3371. doi: 10.1073/pnas.76.7.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonagh R. P., McDonagh J., Petersen T. E., Thøgersen H. C., Skorstengaard K., Sottrup-Jensen L., Magnusson S., Dell A., Morris H. R. Amino acid sequence of the factor XIIIa acceptor site in bovine plasma fibronectin. FEBS Lett. 1981 May 18;127(2):174–178. doi: 10.1016/0014-5793(81)80198-6. [DOI] [PubMed] [Google Scholar]
- McDonald J. A., Kelley D. G. Degradation of fibronectin by human leukocyte elastase. Release of biologically active fragments. J Biol Chem. 1980 Sep 25;255(18):8848–8858. [PubMed] [Google Scholar]
- Menzel E. J., Smolen J. S., Liotta L., Reid K. B. Interaction of fibronectin with C1q and its collagen-like fragment (CLF). FEBS Lett. 1981 Jun 29;129(1):188–192. doi: 10.1016/0014-5793(81)80787-9. [DOI] [PubMed] [Google Scholar]
- Mosesson M. W., Amrani D. L. The structure and biologic activities of plasma fibronectin. Blood. 1980 Aug;56(2):145–158. [PubMed] [Google Scholar]
- Mosesson M. W., Chen A. B., Huseby R. M. The cold-insoluble globulin of human plasma: studies of its essential structural features. Biochim Biophys Acta. 1975 Apr 29;386(2):509–524. doi: 10.1016/0005-2795(75)90294-9. [DOI] [PubMed] [Google Scholar]
- Mosher D. F. Cross-linking of cold-insoluble globulin by fibrin-stabilizing factor. J Biol Chem. 1975 Aug 25;250(16):6614–6621. [PubMed] [Google Scholar]
- Mosher D. F. Fibronectin. Prog Hemost Thromb. 1980;5:111–151. [PubMed] [Google Scholar]
- Mosher D. F., Proctor R. A. Binding and factor XIIIa-mediated cross-linking of a 27-kilodalton fragment of fibronectin to Staphylococcus aureus. Science. 1980 Aug 22;209(4459):927–929. doi: 10.1126/science.7403857. [DOI] [PubMed] [Google Scholar]
- Mosher D. F., Schad P. E., Vann J. M. Cross-linking of collagen and fibronectin by factor XIIIa. Localization of participating glutaminyl residues to a tryptic fragment of fibronectin. J Biol Chem. 1980 Feb 10;255(3):1181–1188. [PubMed] [Google Scholar]
- Pearlstein E., Gold L. I., Garcia-Pardo A. Fibronectin: a review of its structure and biological activity. Mol Cell Biochem. 1980 Feb 8;29(2):103–128. doi: 10.1007/BF00220304. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Vaheri A. Interaction of soluble fibroblast surface antigen with fribrinogen and fibrin. J Exp Med. 1975 Feb 1;141(2):497–501. doi: 10.1084/jem.141.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi K., Fukuda M., Hakomori S. Domain structure of hamster plasma fibronectin. Isolation and characterization of four functionally distinct domains and their unequal distribution between two subunit polypeptides. J Biol Chem. 1981 Jun 25;256(12):6452–6462. [PubMed] [Google Scholar]
- Stemberger A., Hörmann H. Affinity chromatography on immobilized fibrinogen and fibrin monomer, II. The behavior of cold-insoluble globulin [1]. Hoppe Seylers Z Physiol Chem. 1976 Jul;357(7):1003–1005. [PubMed] [Google Scholar]
- Vartio T., Seppä H., Vaheri A. Susceptibility of soluble and matrix fibronectins to degradation by tissue proteinases, mast cell chymase and cathepsin G. J Biol Chem. 1981 Jan 10;256(1):471–477. [PubMed] [Google Scholar]
- Wagner D. D., Hynes R. O. Domain structure of fibronectin and its relation to function. Disulfides and sulfhydryl groups. J Biol Chem. 1979 Jul 25;254(14):6746–6754. [PubMed] [Google Scholar]
- Yamada K. M., Kennedy D. W., Kimata K., Pratt R. M. Characterization of fibronectin interactions with glycosaminoglycans and identification of active proteolytic fragments. J Biol Chem. 1980 Jul 10;255(13):6055–6063. [PubMed] [Google Scholar]
- Zardi L., Siri A., Carnemolla B., Santi L., Gardner W. D., Hoch S. O. Fibronectin: a chromatin-associated protein? Cell. 1979 Nov;18(3):649–657. doi: 10.1016/0092-8674(79)90120-x. [DOI] [PubMed] [Google Scholar]
- de Beer F. C., Baltz M. L., Holford S., Feinstein A., Pepys M. B. Fibronectin and C4-binding protein are selectively bound by aggregated amyloid P component. J Exp Med. 1981 Oct 1;154(4):1134–1139. doi: 10.1084/jem.154.4.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]


