Abstract
Benzo[a]pyrene (BaP) is a ubiquitously distributed environmental pollutant that induces deoxyribonucleic acid (DNA) damage. The inducible heat shock protein (HspA1A) can function as a molecular chaperone; however, its role in DNA repair remains largely unknown. In the present study, human bronchial epithelial cells (16HBE) stably transfected with plasmids carrying HspA1A gene or shRNAs against HspA1A were treated with BaP. DNA damage levels of the cells were evaluated by comet assay. Results suggest that HspA1A could protect cells against DNA damage and facilitate the decrease of DNA damage levels during the first 2 h of DNA repair. DNA repair capacity (DRC) of Benzo(a)pyrene diol epoxide (BPDE)-DNA adducts was evaluated by host cell reactivation assay in the stable 16HBE cells transfected with luciferase reporter vector PCMVluc pretreated with BPDE. Compared with control cells, cells overexpressing HspA1A showed higher DRC (p < 0.01 at 10 μM BPDE and p < 0.05 at 20 μM BPDE, respectively), while knockdown of HspA1A inhibited DNA repair (p < 0.05 at 10 μM BPDE). Moreover, casein kinase 2 (CK2) was shown to interact with HspA1A by mass spectrometry and co-immunoprecipitation assays. The two proteins were co-localized in the cell nucleus and perinuclear region during DNA repair, and were identified by confocal laser scanning microscope. In addition, cells overexpressing HspA1A showed an increased CK2 activity after BaP treatment compared with control cells (p < 0.01). Our results suggest that HspA1A facilitates DNA repair after BaP treatment. HspA1A also interacts with CK2 and enhances the kinase activities of CK2 during DNA repair.
Electronic supplementary material
The online version of this article (doi:10.1007/s12192-013-0454-7) contains supplementary material, which is available to authorized users.
Keywords: HspA1A, DNA repair, Casein kinase 2, Benzo[a]pyrene
Introduction
The need for organisms to withstand sudden stressful changes has led to the evolution of a variety of adaptive pathways including the heat shock response. The inducible heat shock protein (HspA1A) of mammalian cells is one of the most abundant heat shock protein families, and it mainly contributes to the cytoprotection against a variety of noxious events (Bukau and Horwich 1998; Lindquist and Craig 1988).
HspA1A functions as a molecular chaperone in physiological conditions guiding protein folding (Beckmann et al. 1990), translocation (Chirico et al. 1988) and degradation (Chiang et al. 1989). When stressful conditions prevail, HspA1A can aid the misfolded proteins to reconstitute normal three-dimensional structures. HspA1A also protects cells and tissues against deoxyribonucleic acid (DNA) damage caused by a wide spectrum of stressful stimuli (Hightower 1991; Niu et al. 2006; Wu et al. 1996, 2001). Moreover, the expression level of HspA1A is inversely correlated with DNA damage levels in lymphocytes of coke oven workers (Xiao et al. 2002), hinting that HspA1A may be involved in DNA damage and repair.
Accumulating evidence has demonstrated the role of HspA1A in DNA repair including the base excision repair (BER) and nucleotide excision repair (NER) pathways. HspA1A can bind to human apurinic/apyrimidinic endonuclease and stimulate endonuclease activities at abasic sites (Kenny et al. 2001). HspA1A also stimulates single-strand gap-filling by interacting with DNA polymerase beta (Mendez et al. 2003). In heat shock treated HeLa cells, HspA1A protects cells from single-strand DNA breaks by translocating to the cell nuclei/nucleoli and binding to XRCC1 and PARP-1 (Kotoglou et al. 2009). HspA1A also enhances deoxyribonucleic acid BER in human leukemic cells after ionizing radiation (Bases 2006). DnaK, the bacterial homolog of HspA1A, maintains the properly folded state of DNA repair proteins and participates in NER (Zou et al. 1998). One of our previous studies also identified an increased co-localization between HspA1A and the NER proteins XPA and XPG in cell nucleus after exposure to Benzo[a]pyrene (BaP) (Yang et al. 2009). However, the underlying mechanism of how HspA1A facilitates DNA repair remains elusive.
To gain a global view of the biological functions that HspA1A may exert in DNA repair, stably transfected human bronchial epithelial (16HBE) cells and BaP were applied as the experimental model and stress stimulus, respectively. DNA damages caused by BaP are mainly repaired through the NER and BER pathways (Celotti et al. 1993; Fleck and Nielsen 2004). Cellular DNA damage levels were evaluated by comet assay at different time points after BaP treatment. The DNA repair capacity (DRC) of BPDE-DNA adducts was also assessed by the modified host cell reactivation (HCR) assay (Ahn et al. 2004) in stable 16HBE cells transfected with luciferase reporter vector PCMVluc damaged by Benzo(a)pyrene diol epoxide (BPDE), one metabolite of BaP. Liquid chromatography-electrospray ionization tandem mass spectrometry (LC-ESI MS) and co-immunoprecipitation (Co-IP) assays were applied to identify proteins that interacted with HspA1A. Cellular localization of the two proteins in cells after BaP exposure was evaluated by confocal laser scanning microscopy. In addition, the kinase activity of CK2 was also assessed in the stably transfected 16HBE cells during DNA repair.
Materials and methods
Cell culture and treatment
The immortalized 16HBE cell line, kindly provided by Dr. Y. Jiang (Guangzhou Medical College, China), was cultured in minimal essential medium (GIBCO, USA). HEK293 cells were kindly provided by Dr. J. Wang (Tongji Medical College, China) and cultured in Dulbecco modified Eagle medium (GIBCO). Both cells were supplemented with 10 % fetal calf serum (GIBCO) and cultured in a humidified atmosphere containing 5 % CO2 at 37 °C. 16HBE cells were exposed to BaP as previously described (Yang et al. 2007). All experiments were conducted with cells at a logarithmic stage of growth curve.
Chemicals and plasmids
BaP and BPDE were purchased from the National Cancer Institute (NCI, USA). Lipofectamine 2000 and the plasmid PCMV-Myc were obtained from Invitrogen. The firefly luciferase reporter plasmid PCMVluc was kindly provided by Dr. Q. Wei (The University of Texas, MD Anderson Cancer Center, USA). PcDNA3.1-HspA1A, pcDNA3.1 and pcDNA3-Flag were kindly provided by Dr. X. Xiao (Central South University, China). PSilencer 2.1-U6 neo carrying shRNA against HspA1A or control shRNAs were purchased from Ambion. Full length of HspA1A was amplified from pcDNA3.1-HspA1A and subsequently in-frame cloned into pcDNA3-Flag. CK2α was amplified from the human genome and cloned into pCMV-Myc. Information about primers was shown in Supplementary Table 1.
Stable transfection
To generate cells stably expressing high or low levels of HspA1A, the immortalized 16HBE cells were transfected with pcDNA3.1, pcDNA3.1-HspA1A, shRNA-control or shRNA-HspA1A using lipofectamine 2000 (Invitrogen). After transfection for 48 h, cells were selected with G418 (800 μg/l) for 3 weeks. The expression levels of HspA1A in the surviving colonies were analyzed by immunofluorescence microscopy and immunoblotting.
Treatment of 16HBE cells with heat shock
To better understand the accumulation or reduction of HspA1A, 16HBE cells and cells stably transfected with shRNA-HspA1A were heated at 42 °C for 1 h and then recovered at 37 °C for 24 h. Cells were harvested for analysis of protein levels HspA1A.
Immunofluorescence microscopy
The stably transfected 16HBE cells were fixed with acetone for 15 min, washed three times with phosphate buffered saline (PBS) and then permeabilized with 0.5 % Triton-X-100 for 15 min. Non-specific binding sites were blocked with 10 % bovine serum albumin (BSA) for 30 min at room temperature followed by an incubation with rabbit anti-HspA1A primary antibody (#SPA-812, Stressgen, Victoria, BC, Canada) at 37 °C for 2 h. After three washes with PBS, cells were then incubated with goat anti-rabbit immunoglobulin G (IgG) secondary antibody conjugated with FITC (#4030-02, Southern Biotech, USA) at 37 °C for 1 h. Images were analyzed by a fluorescence microscope (Olympus B-60F5, Japan).
Western blotting
16HBE cells were lysed in Triton X-100 buffer (P0013, Beyotime Institute of Biotechnology, China) with protease inhibitors. About 35 μg proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and blotted onto nitrocellulose membranes. After blocking with 5 % non-fat milk for 1 h at room temperature, the membranes were incubated with mouse anti-HspA1A (#SPA-810, Stressgen) or mouse anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH, KC-5G4, KangCheng, Shanghai, China) primary antibody overnight at 4 °C followed by an incubation with goat anti-mouse (#6170-05, Southern Biotech) IgG secondary antibodies conjugated with horseradish peroxidase (HRP) at room temperature for 2 h. Proteins were visualized by an enhanced chemiluminescences detection kit (Amersham Bioscience, UK).
Comet assay
DNA damage levels induced by BaP exposure were detected by the alkaline comet assay as described previously (Singh et al. 1988). Images were analyzed by the IBM-compatible personal computer-based image analysis system IMI 1.0. DNA damage levels were expressed as Olive Tail Moment (OTM) values.
Host cell reactivation assay
Luciferase reporter plasmid PCMVluc was incubated with different doses of BPDE (0, 10, 20, 30, or 40 μM) as previously described (Ahn et al. 2004) to generate BPDE-DNA adducts before they were transfected into 16HBE cells. Efficient repair of the BPDE-DNA adducts can induce the expression of luciferase genes. After 48 h of transfection, the luminescent signal from the luciferase reaction was measured by the luciferase assay system (E1500, Promega, USA) and a single sample illuminometer (TD-20/20 DLReasy, Promega).
Mass spectrometry
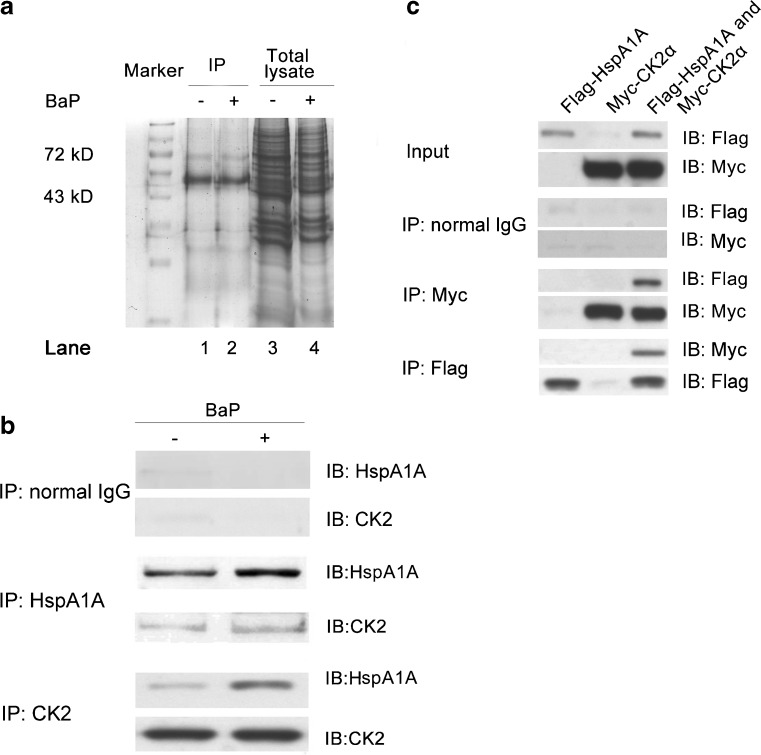
16HBE cells were lysed in IP lysis buffer (50 mM Tris–HCl pH 7.4, 150 mM NaCl, 0.1 % SDS, 1 % NP-40, 0.5 % Sodium deoxycholate) with a mixture of protease inhibitors (2 μg/ml of leupeptin and aprotinin, and 1 mM PMSF) on ice for 45 min. Cell lysates were centrifuged at 10,000 × g for 10 min at 4 °C, and 900 μg proteins in the supernatant were incubated with 2 μg rabbit normal IgG (#SC-2027, Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4 °C for 2 h followed by an incubation with 30 μl protein A/G-agarose beads (Stressgen) at 4 °C for 1 h to pre-clear the non-specific immunocomplexes. After centrifugation for 10 min at 1000 g, the supernatants were further incubated with 2 μg rabbit anti-HspA1A primary antibody (#SPA-812, Stressgen) overnight at 4 °C. The immunocomplexes were washed four times with lysis buffer after capture on protein A/G-agarose beads. The bound proteins were eluted with 1 × SDS sample buffer and denatured at 95 °C for 5 min. The precipitates were separated by SDS-PAGE and stained with Coomassie brilliant blue. Lane 2 proteins (Fig. 3a) were analyzed by LC-ESI MS at the Research Centre for Proteome Analysis (RCPA, Chinese Academy of Sciences in Shanghai) as described previously (He et al. 2005).
Fig. 3.
Interaction between HspA1A and CK2. a Normally cultured 16HBE cells were treated with or without 16 μM BaP for 2 h and recovered for 4 h. A total of 900 μg proteins were immunoprecipitated (IP) with HspA1A-specific antibody. Precipitates (lanes 1, 2) and the input lysates (lanes 3, 4) were separated by SDS-polyacrylamide gel electrophoresis. Lane 2 proteins were analyzed by liquid chromatography-electrospray ionization tandem mass spectrometry. b 16HBE cells were treated with or without 16 μM BaP for 2 h and recovered for 4 h. A total of 900 μg proteins were immunoprecipitated with 2 μg rabbit normal IgG (upper panel), 2 μg HspA1A (middle panel) or 2 μg CK2 antibody (lower panel) overnight at 4 °C. Precipitates were detected by immunoblotting (IB) with antibodies against CK2 or HspA1A, respectively. c HEK293 cells (5 × 105 cells per well) were transfected with 10 μg of Flag-HspA1A or/and 10 μg of Myc-CK2α for 48 h. The input lysates were immunoblotted with Flag and Myc antibodies (upper panel) and were immunoprecipitated with normal IgG (second panel), Myc (third panel) or Flag (low panel) antibodies
Co-immunoprecipitation
The pre-clearance of non-specific immunocomplexes was performed using rabbit normal IgG (#SC-2027, Santa Cruz Biotechnology) or goat normal IgG (A7007, Beyotime Institute of Biotechnology). Immunoprecipitation was conducted using 2 μg rabbit normal IgG, 2 μg rabbit anti-HspA1A antibody (#SPA-812, Stressgen) or 2 μg goat anti-CK2 antibody (sc-6479, Santa Cruz Biotechnology) respectively. Beads were washed and detected with mouse anti-HspA1A (#SPA-810, Stressgen) or goat anti-CK2 (sc-6479, Santa Cruz Biotechnology) primary antibodies followed by an incubation with the goat anti-mouse (#6170-05, Southern Biotech) or rabbit anti-goat (#6160-05, Southern Biotech) IgG secondary antibody conjugated with HRP. The rabbit normal IgG, mouse anti-Myc (AB103, Tiangen Biotech, China) and rabbit anti-Flag (#2368, Cell Signaling Technology, USA) primary antibody were applied in the exogenous Co-IP assay. The bound proteins were detected by immunoblotting using the rabbit anti-Flag or mouse anti-Myc primary antibodies, respectively.
Confocal laser scanning microscopy
Cells grown on cover slips were fixed in acetone for 15 min at room temperature. After wash, they were permeabilized and blocked in buffer containing 0.5 % Triton X-100 and 10 % BSA for 30 min. Cells were subsequently incubated with rabbit anti-HspA1A antibody (#SPA-812, Stressgen) and goat anti-CK2 primary antibody (sc-6479, Santa Cruz Biotechnology) overnight at 4 °C, then with goat anti-rabbit IgG secondary antibody conjugated with FITC (#4030-02, Southern Biotech) and rabbit anti-goat IgG secondary antibody conjugated with Rhodamine (#6160-03, Southern Biotech). Images were analyzed on a laser-scanning confocal microscope (LSM510, Carl Zeiss, Germany).
Detection of CK2 activity
The standard assay for phosphotransferase activity of CK2 was conducted in a reaction mixture containing 20 mM Tris–HCl (pH 7.5), 120 mM KCl, 10 mM MgCl2, and 100 mM [γ-32P] ATP in the presence of 1 mM synthetic peptide substrate (RRREEETEEE) in a total volume of 30 μl at 30 °C. The reactions were started by the addition of 16HBE cell lysates and incubated for 15 min. The reaction was stopped after the addition of trichloroacetic acid to a final concentration of 10 %, and 10 μl of supernatant was then applied to P-81 paper. The paper was washed in 100 mM phosphoric acid and measured for the radioactivity by scintillation counting.
Statistical analysis
The difference in HspA1A protein levels, OTM values, luciferase activities, and the CK2 activities between groups of cells was examined by one-way analysis of variance. All statistical analyses were performed using SPSS 11.0 software (Statistical Package for the Social Sciences, Chicago, USA). A value of p < 0.05 was considered to be significant (two-tailed).
Results
The expression levels of HspA1A in 16HBE cells after transfection
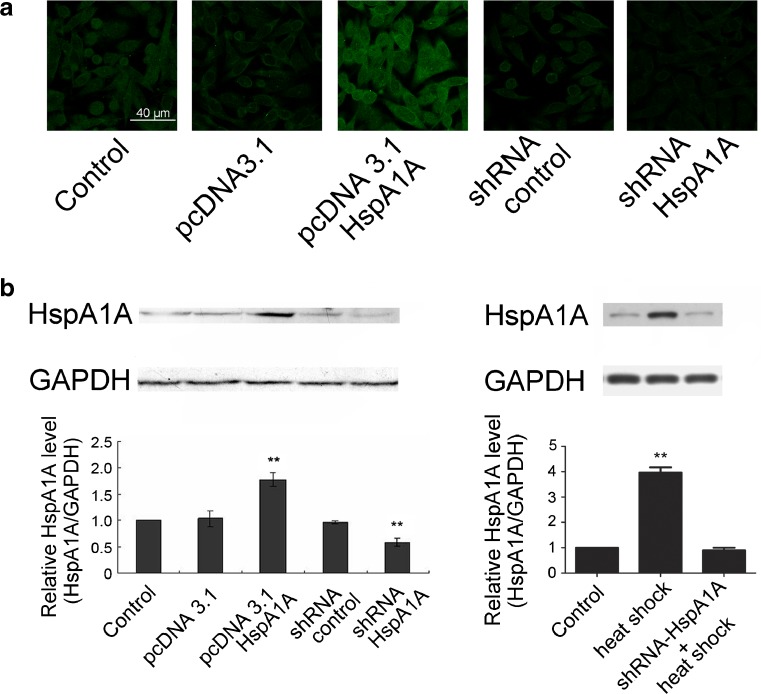
Expression levels of HspA1A in the stably transfected 16HBE cells were examined by immunofluorescence microscopy and immunoblotting. Cells transfected with pcDNA3.1-HspA1A showed higher fluorescence intensity compared with the control cells, while the fluorescence intensity in shRNA-HspA1A transfected cells decreased (Fig.1a). Quantification of the immunoblotting results demonstrated a twofold increase in HspA1A protein levels in cells transfected with pcDNA3.1-HspA1A, while the level decreased to 40–50 % in cells transfected with shRNA-HspA1A (Fig.1b, left). No significant difference was found between pcDNA3.1, shRNA-control transfected cells and the control cells. HspA1A levels in the 16HBE cells treated with heat shock increased to about fourfold compared with the control, while no significant difference was found between the control and the HspA1A knockdown cells (Fig.1b, right).
Fig. 1.
The expression level of HspA1A in 16HBE cells. The immortalized 16HBE cells were stably transfected with pcDNA3.1, pcDNA3.1-HspA1A, shRNA-control or shRNA-HspA1A, respectively. Normal cultured 16HBE cells served as the control. a Cells were immunostained with antibody to HspA1A and FITC-labeled anti-rabbit secondary antibody (green). b Total protein lysates were analyzed for HspA1A protein level by Western blotting. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as the loading control. Quantifications of immunoblotting are expressed as mean ± SD from three independent experiments. **p < 0.01 compared with the control
HspA1A protected cells against DNA damage induced by BaP treatment and could facilitate DNA repair
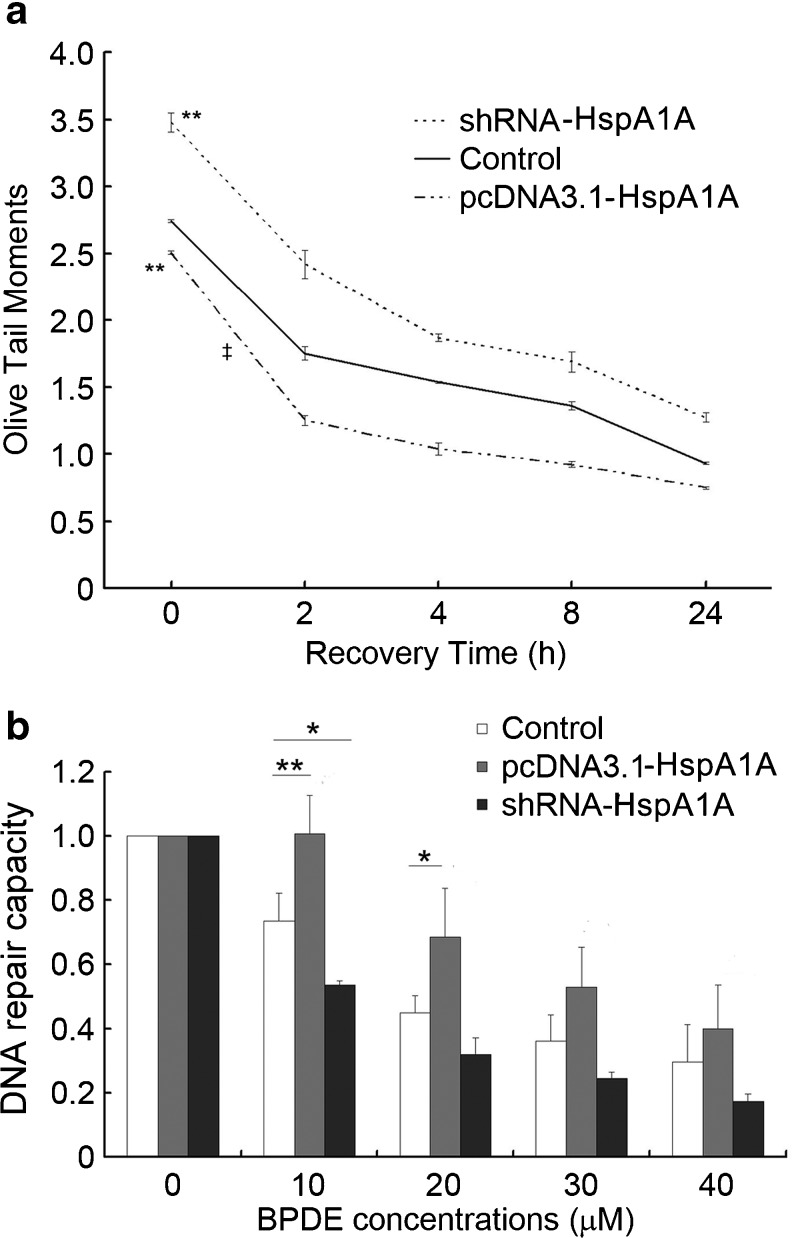
The stably transfected 16HBE cells were treated with BaP for 2 h and recovered for 0–24 h. DNA damage levels were examined at indicated time points. Cell viabilities were above 80 % in all the cell groups (data not shown). The OTM value was significantly higher (p < 0.01) in cells after knockdown of HspA1A expression compared with the control cells at 0 h, while it was significantly lower (p < 0.01) in cells overexpressing HspA1A (Fig. 2a). Moreover, compared with the control, cells overexpressing HspA1A showed a more efficient reduction in DNA damage levels in the first 2 h (the OTM value at 2 h minus the OTM value at 0 h, p < 0.01), while the reduction efficiency is similar between the HspA1A knockdown cells and control cells at 2 h.
Fig. 2.
HspA1A facilitates repair of DNA damage induced by Benzo[a]pyrene (BaP) and Benzo(a)pyrene diol epoxide (BPDE). a The 16HBE cells were treated with 16 μM BaP for 2 h and recovered for 0, 2, 4, 8 and 24 h. Results are expressed as mean ± SD from three independent experiments. **p < 0.01 compared with the control at 0 h; ‡p < 0.01 compared with the control in the first 2 h of repair. b The firefly luciferase reporter plasmids PCMVluc were incubated with various doses of BPDE for 3 h at room temperature before they were transfected into 16HBE cells. After 48 h of transfection, the luciferase activities of the cell lysates were analyzed. DNA repair capacity was calculated as the percentage of luciferase activity from the damaged reporter to that of the unmodified reporter. Results are expressed as mean ± SD from three independent experiments. *p < 0.05, **p < 0.01 compared with the corresponding control
HspA1A facilitated removal of BPDE-DNA adducts
To investigate whether HspA1A was involved in NER, the DRC of BPDE-DNA adducts was evaluated by HCR assay. As shown in Fig.2b, the DNA repair capacities were consistently higher in cells overexpressing HspA1A compared with the corresponding control cells (p < 0.01 at 10 μM BPDE; p < 0.05 at 20 μM BPDE). In contrast, knockdown of HspA1A expression led to a lower luciferase activity (p < 0.05 at 10 μM BPDE). No significant difference was found among the three groups of cells at the 30 or 40 μM BPDE group.
Interaction between HspA1A and CK2
We then aimed to discover proteins that might interact with HspA1A. After immunoprecipitation with anti-HspA1A primary antibody, HspA1A (molecular weight 72 kDa) was readily detectable in the precipitates from cells with or without BaP exposure (Fig. 3a, lanes 1 and 2). A total of 84 proteins were shown to interact with HspA1A (Supplementary Table 2 and Supplementary Fig. 1) during DNA repair. We focused our further study on the interaction between HspA1A and CK2, which is a pleiotropic protein relevant to DNA repair (Olsen et al. 2012). The interaction between CK2 and HspA1A was confirmed by endogenous and exogenous Co-IP assays. As shown in Fig. 3b, CK2 was detectable in precipitates of cells without BaP treatment after IP with HspA1A antibody (middle panel). HspA1A was also detectable in precipitates of cells without BaP treatment after IP with CK2 antibody (lower panel). Of note, the binding between HspA1A and CK2 was increased after BaP treatment (Fig. 3b). Neither of the two proteins was detectable in the beads precipitated by normal IgG (upper panel). Flag-HspA1A was immunoprecipitated with Myc-CK2α in cells transfected with both HspA1A and CK2α, but not in cells transfected with only one of the two proteins (Fig. 3c).
Co-localization between HspA1A and CK2 in the cell nucleus and perinuclear region after BaP treatment
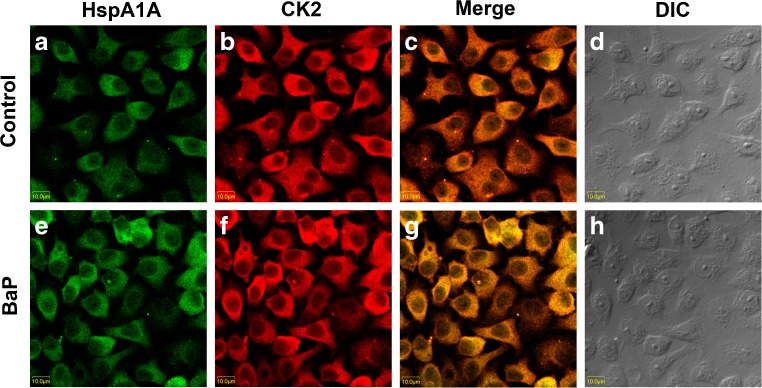
Sub-cellular locations of HspA1A and CK2 were analyzed in 16HBE cells with or without BaP treatment by confocal immunofluorescence microscopy. As shown in Fig. 4, HspA1A and CK2 were homogenously distributed and overlapped at some areas in the cytoplasm (Fig. 4a, b, c) in cells without BaP treatment. After BaP treatment and recovery for 4 h, the fluorescence intensities of the two proteins in cells both increased (Fig. 4e, f). Of note, the two proteins were readily detectable and overlapped at some areas in the cell nucleus and perinuclear region (Fig. 4g). The images analyzed by differential interference contrast microscopy represented the fluorescence label-free cells (Fig. 4d, h).
Fig. 4.
Co-localization of HspA1A and CK2 in cell nucleus and perinuclear region during DNA repair. Normally cultured 16HBE cells were treated with or without 16 μM BaP for 2 h and recovered for 4 h. The cells were incubated with anti-HspA1A antibody (a, e) or anti-CK2 antibody (b, f) to detect the subcellular location of the two proteins by con-focal laser scanning microscopy. Overlay of the immunoreactions are shown in the merged images (c, g). Differential interference contrast (DIC) microscopy was used to analyze the fluorescence label-free cells (d, h)
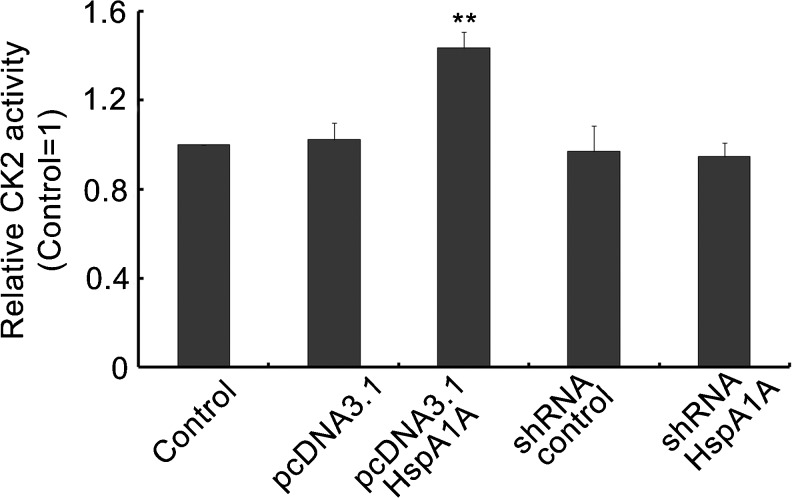
Over-expression of HspA1A enhanced CK2 activity during DNA repair
HspA1A might also regulate the activity of CK2 during DNA repair. As shown in Fig. 5, CK2 activity was increased in cells overexpressing HspA1A compared with the control cells (p < 0.01). No significant difference was found between cells transfected with pcDNA3.1, shRNA-control, shRNA-HspA1A, and the control cells.
Fig. 5.
High levels of HspA1A enhanced CK2 activity. The stably transfected 16HBE cells were exposed to 16 μM BaP for 2 h and recovered for 4 h. Results are expressed as mean ± SD from three independent experiments. **p < 0.01 compared with the control
Discussion
The aim of the present study was to explore the roles of HspA1A in DNA repair. We found that HspA1A could protect cells against DNA damage induced by BaP treatment and facilitate DNA repair including removal of the BPDE-DNA adducts. During DNA repair, HspA1A interacted and co-localized with CK2 in the cell nucleus and perinuclear region, and overexpression of HspA1A also enhanced the kinase activity of CK2.
The carcinogenesis of BaP depends on its ultimate metabolites BPDE and reactive oxygen species, and BaP exposure by inhalation can cause genetic damage to human lung cells. Therefore, the human bronchial epithelial cell was used as the experimental model in our study. DNA damage induced by BaP treatment can be repaired by the NER and BER pathways (Celotti et al. 1993; Fleck and Nielsen 2004). HSPs associate with the BER enzymes and regulate their functions in HeLa cells (Mendez et al. 2000). HspA1A also co-localizes with the NER proteins in cell nuclei after BaP exposure, such as XPA and XPG (Yang et al. 2009). In our study, we found that the DNA damage levels decreased most efficiently in cells overexpressing HspA1A in the first 2 h of DNA repair. BPDE-DNA adducts are mainly repaired by activation of the NER pathway (Celotti et al. 1993), and our study provides direct evidence showing the role of HspA1A in repair of BPDE-DNA adducts by HCR assay, hinting at the involvement of HspA1A in the NER pathway. When the reporter plasmid was treated with 10 μM BPDE, HspA1A knockdown inhibited DRC of the BPDE-DNA adducts, whereas the DRC showed no significant difference between the control and HspA1A knockdown cells treated with other concentrations of BPDE. Likewise, the reduction efficiency is similar between HspA1A knockdown cells and control cells at 2 h (Fig. 2a), which might be due to the compensation of other DNA repair enzymes involved in the DNA repair process. Alternatively, the extent of HspA1A knockdown might not be sufficient to influence the DRC in cells treated with the concentrations of BaP or BPDE we used in this study.
Nuclear transportation is a fundamental but critical mechanism regulating protein localization and function (Hood and Silver 2000; Knudsen et al. 2009). HspA1A translocates and accumulates in the cell nucleus under heat shock or other stressful conditions (Lepock et al. 2001; Szekely et al. 1995). One recent study demonstrates that HspA1A can protect HeLa cells from single-strand DNA breaks by translocating to the cell nucleus and interacting with XRCC1 and PARP-1 (Kotoglou et al. 2009), which are two critical proteins involved in DNA repair. We found that HspA1A interacted and co-localized with CK2 in the cell nucleus and perinuclear region, implying a potential translocation of the two proteins to the cell nucleus during DNA repair. CK2 is a multifunctional protein involved in cell differentiation, proliferation, and survival (Ahmed et al. 2002; Litchfield 2003). CK2 also targets many substrates in response to stress stimuli such as heat shock, ultraviolet radiation, hypoxia, DNA damage, and viral infections (Filhol and Cochet 2009). DNA repair can be facilitated by CK2 through constitutively phosphorylating XRCC1 (Loizou et al. 2004; Parsons et al. 2010) and regulating the expression of Rad51 (Yata et al. 2012). In addition, CK2 can modulate the activity of the DNA-dependent protein kinase (DNA-PK), which is a major component of the non-homologous end-joining pathway of DNA double-strand breaks repair (Olsen et al. 2010). Moreover, we found that CK2 activity was significantly increased during DNA repair with BaP exposure in cells overexpressing HspA1A. The recombinant heat shock protein 90 (Hsp90) also interacts with CK2 and enhances its activity in Escherichia coli (Shi et al. 1994). No significant change in CK2 activity was found in cells transfected with shRNA-HspA1A compared with the control, which may be because the extent of HspA1A knockdown in our study is not sufficient to affect the CK2 activity. Alternatively, the compensation of CK2 activities might be invoked when HspA1A expression was knocked down.
Two major limitations should be mentioned in this study. First, the effect of modulated CK2 expression or activity on DNA repair was not analyzed, although we can speculate from the existing data that HspA1A might function as an upstream regulator of CK2 in the DNA repair pathway. Second, the findings in our study need to be validated in more cell lines.
In conclusion, our study indicates that HspA1A can facilitate the repair of BaP-induced DNA damage including removal of BPDE-DNA adducts. In addition, HspA1A interacted and co-localized with CK2 in the cell nucleus and perinuclear region during DNA repair, and overexpression of HspA1A also enhanced the activity of CK2 during DNA repair after BaP treatment. Further experiments are required to demonstrate the biological relevance of the interaction between HspA1A and CK2 during DNA repair.
Electronic supplementary material
(DOC 462 kb)
Acknowledgments
This work was supported by the National Outstanding Youth Science Foundation of China [30525031] and the National Natural Science Foundation of China [30872092].
Footnotes
Yanying Duan and Suli Huang contributed equally to this work
References
- Ahmed K, Gerber DA, Cochet C. Joining the cell survival squad: an emerging role for protein kinase CK2. Trends Cell Biol. 2002;12:226–230. doi: 10.1016/S0962-8924(02)02279-1. [DOI] [PubMed] [Google Scholar]
- Ahn B, Kang D, Kim H, Wei Q. Repair of mitomycin C cross-linked DNA in mammalian cells measured by a host cell reactivation assay. Mol Cells. 2004;18:249–255. [PubMed] [Google Scholar]
- Bases R. Heat shock protein 70 enhanced deoxyribonucleic acid base excision repair in human leukemic cells after ionizing radiation. Cell Stress Chaperones. 2006;11:240–249. doi: 10.1379/CSC-185R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann RP, Mizzen LE, Welch WJ. Interaction of Hsp 70 with newly synthesized proteins: implications for protein folding and assembly. Science. 1990;248:850–854. doi: 10.1126/science.2188360. [DOI] [PubMed] [Google Scholar]
- Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. doi: 10.1016/S0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- Celotti L, Ferraro P, Furlan D, Zanesi N, Pavanello S. DNA repair in human lymphocytes treated in vitro with (+)-anti- and (+/-)-syn-benzo[a]pyrene diolepoxide. Mutat Res. 1993;294:117–126. doi: 10.1016/0921-8777(93)90020-H. [DOI] [PubMed] [Google Scholar]
- Chiang HL, Terlecky SR, Plant CP, Dice JF. A role for a 70-kilodalton heat shock protein in lysosomal degradation of intracellular proteins. Science. 1989;246:382–385. doi: 10.1126/science.2799391. [DOI] [PubMed] [Google Scholar]
- Chirico WJ, Waters MG, Blobel G. 70K heat shock related proteins stimulate protein translocation into microsomes. Nature. 1988;332:805–810. doi: 10.1038/332805a0. [DOI] [PubMed] [Google Scholar]
- Filhol O, Cochet C. Protein kinase CK2 in health and disease: cellular functions of protein kinase CK2: a dynamic affair. Cell Mol Life Sci. 2009;66:1830–1839. doi: 10.1007/s00018-009-9151-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleck O, Nielsen O. DNA repair. J Cell Sci. 2004;117:515–517. doi: 10.1242/jcs.00952. [DOI] [PubMed] [Google Scholar]
- He P, He HZ, Dai J, et al. The human plasma proteome: analysis of Chinese serum using shotgun strategy. Proteomics. 2005;5:3442–3453. doi: 10.1002/pmic.200401301. [DOI] [PubMed] [Google Scholar]
- Hightower LE. Heat shock, stress proteins, chaperones, and proteotoxicity. Cell. 1991;66:191–197. doi: 10.1016/0092-8674(91)90611-2. [DOI] [PubMed] [Google Scholar]
- Hood JK, Silver PA. Diverse nuclear transport pathways regulate cell proliferation and oncogenesis. Biochim Biophys Acta. 2000;1471:M31–M41. doi: 10.1016/s0304-419x(00)00018-4. [DOI] [PubMed] [Google Scholar]
- Kenny MK, Mendez F, Sandigursky M, Kureekattil RP, Goldman JD, Franklin WA, Bases R. Heat shock protein 70 binds to human apurinic/apyrimidinic endonuclease and stimulates endonuclease activity at abasic sites. J Biol Chem. 2001;276:9532–9536. doi: 10.1074/jbc.M009297200. [DOI] [PubMed] [Google Scholar]
- Knudsen NO, Andersen SD, Lutzen A, Nielsen FC, Rasmussen LJ. Nuclear translocation contributes to regulation of DNA excision repair activities. DNA Repair (Amst) 2009;8:682–689. doi: 10.1016/j.dnarep.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Kotoglou P, Kalaitzakis A, Vezyraki P, Tzavaras T, Michalis LK, Dantzer F, Jung JU, Angelidis C. Hsp70 translocates to the nuclei and nucleoli, binds to XRCC1 and PARP-1, and protects HeLa cells from single-strand DNA breaks. Cell Stress Chaperones. 2009;14:391–406. doi: 10.1007/s12192-008-0093-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepock JR, Frey HE, Heynen ML, Senisterra GA, Warters RL. The nuclear matrix is a thermolabile cellular structure. Cell Stress Chaperones. 2001;6:136–147. doi: 10.1379/1466-1268(2001)006<0136:TNMIAT>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Litchfield DW. Protein kinase CK2: structure, regulation and role in cellular decisions of life and death. Biochem J. 2003;369:1–15. doi: 10.1042/BJ20021469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loizou JI, El-Khamisy SF, Zlatanou A, et al. The protein kinase CK2 facilitates repair of chromosomal DNA single-strand breaks. Cell. 2004;117:17–28. doi: 10.1016/S0092-8674(04)00206-5. [DOI] [PubMed] [Google Scholar]
- Mendez F, Sandigursky M, Franklin WA, Kenny MK, Kureekattil R, Bases R. Heat-shock proteins associated with base excision repair enzymes in HeLa cells. Radiat Res. 2000;153:186–195. doi: 10.1667/0033-7587(2000)153[0186:HSPAWB]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Mendez F, Kozin E, Bases R. Heat shock protein 70 stimulation of the deoxyribonucleic acid base excision repair enzyme polymerase beta. Cell Stress Chaperones. 2003;8:153–161. doi: 10.1379/1466-1268(2003)008<0153:HSPSOT>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu P, Liu L, Gong Z, Tan H, Wang F, Yuan J, Feng Y, Wei Q, Tanguay RM, Wu T. Overexpressed heat shock protein 70 protects cells against DNA damage caused by ultraviolet C in a dose-dependent manner. Cell Stress Chaperones. 2006;11:162–169. doi: 10.1379/CSC-175R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen BB, Issinger OG, Guerra B. Regulation of DNA-dependent protein kinase by protein kinase CK2 in human glioblastoma cells. Oncogene. 2010;29:6016–6026. doi: 10.1038/onc.2010.337. [DOI] [PubMed] [Google Scholar]
- Olsen BB, Wang SY, Svenstrup TH, Chen BP, Guerra B. Protein kinase CK2 localizes to sites of DNA double-strand break regulating the cellular response to DNA damage. BMC Mol Biol. 2012;13:7. doi: 10.1186/1471-2199-13-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JL, Dianova II, Finch D, Tait PS, Strom CE, Helleday T, Dianov GL. XRCC1 phosphorylation by CK2 is required for its stability and efficient DNA repair. DNA Repair (Amst) 2010;9:835–841. doi: 10.1016/j.dnarep.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Shi Y, Brown ED, Walsh CT. Expression of recombinant human casein kinase II and recombinant heat shock protein 90 in Escherichia coli and characterization of their interactions. Proc Natl Acad Sci U S A. 1994;91:2767–2771. doi: 10.1073/pnas.91.7.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- Szekely L, Jiang WQ, Pokrovskaja K, Wiman KG, Klein G, Ringertz N. Reversible nucleolar translocation of Epstein–Barr virus-encoded EBNA-5 and hsp70 proteins after exposure to heat shock or cell density congestion. J Gen Virol. 1995;76:2423–2432. doi: 10.1099/0022-1317-76-10-2423. [DOI] [PubMed] [Google Scholar]
- Wu TC, Tanguay RM, Wu Y, et al. Presence of antibodies to heat stress proteins and its possible significance in workers exposed to high temperature and carbon monoxide. Biomed Environ Sci. 1996;9:370–379. [PubMed] [Google Scholar]
- Wu T, Chen S, Xiao C, et al. Presence of antibody against the inducible Hsp71 in patients with acute heat-induced illness. Cell Stress Chaperones. 2001;6:113–120. doi: 10.1379/1466-1268(2001)006<0113:POAATI>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Chen S, Li J, et al. Association of HSP70 and genotoxic damage in lymphocytes of workers exposed to coke-oven emission. Cell Stress Chaperones. 2002;7:396–402. doi: 10.1379/1466-1268(2002)007<0396:AOHAGD>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Liu X, Niu P, Zou Y, Gong Z, Yuan J, Wu T. Dynamic changes of XPA, XPC, XPF, XPG and ERCC1 protein expression and their correlations with levels of DNA damage in human bronchial epithelia cells exposed to benzo[a]pyrene. Toxicol Lett. 2007;174:10–17. doi: 10.1016/j.toxlet.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Yang J, Liu X, Niu P, Zou Y, Duan Y. Correlations and co-localizations of Hsp70 with XPA, XPG in human bronchial epithelia cells exposed to benzo[a]pyrene. Toxicology. 2009;265:10–14. doi: 10.1016/j.tox.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Yata K, Lloyd J, Maslen S, Bleuyard JY, Skehel M, Smerdon SJ, Esashi F. Plk1 and CK2 act in concert to regulate Rad51 during DNA double strand break repair. Mol Cell. 2012;45:371–383. doi: 10.1016/j.molcel.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Crowley DJ, Van Houten B. Involvement of molecular chaperonins in nucleotide excision repair. Dnak leads to increased thermal stability of UvrA, catalytic UvrB loading, enhanced repair, and increased UV resistance. J Biol Chem. 1998;273:12887–12892. doi: 10.1074/jbc.273.21.12887. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 462 kb)