Abstract
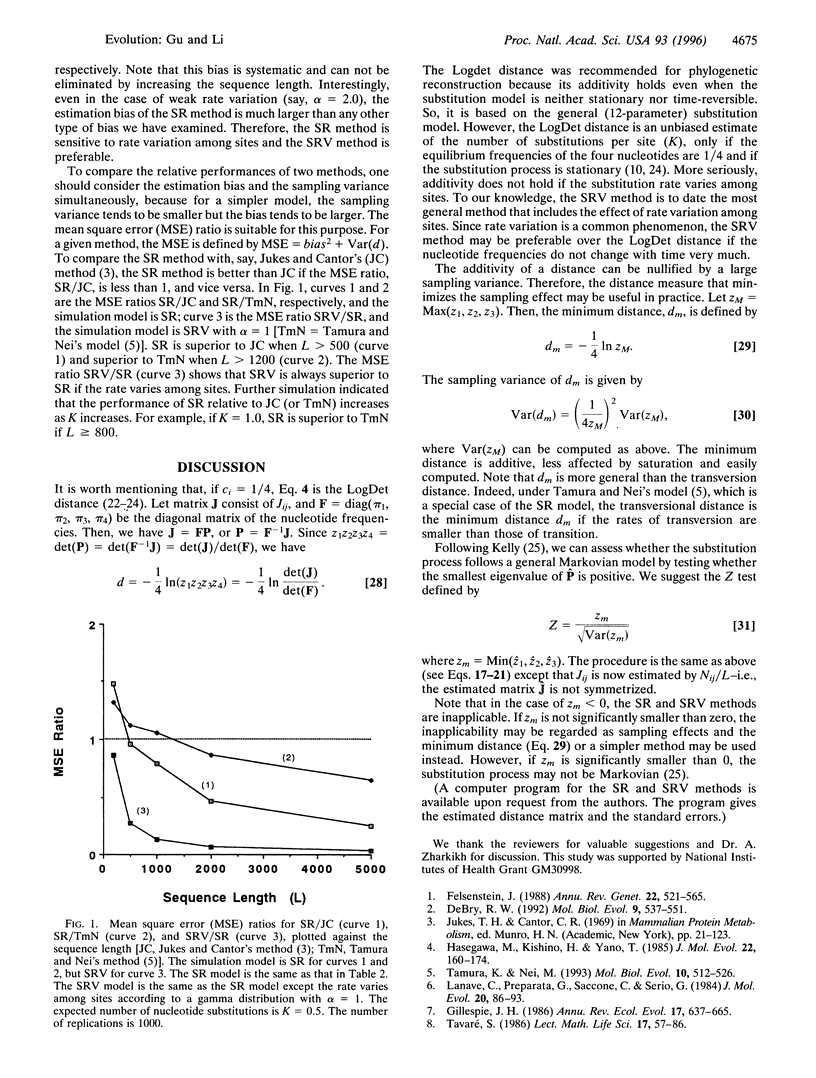
As additivity is a very useful property for a distance measure, a general additive distance is proposed under the stationary time-reversible (SR) model of nucleotide substitution or, more generally, under the stationary, time-reversible, and rate variable (SRV) model, which allows rate variation among nucleotide sites. A method for estimating the mean distance and the sampling variance is developed. In addition, a method is developed for estimating the variance-covariance matrix of distances, which is useful for the statistical test of phylogenies and molecular clocks. Computer simulation shows (i) if the sequences are longer than, say, 1000 bp, the SR method is preferable to simpler methods; (ii) the SR method is robust against deviations from time-reversibility; (iii) when the rate varies among sites, the SRV method is much better than the SR method because the distance is seriously underestimated by the SR method; and (iv) our method for estimating the sampling variance is accurate for sequences longer than 500 bp. Finally, a test is constructed for testing whether DNA evolution follows a general Markovian model.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry D., Hartigan J. A. Asynchronous distance between homologous DNA sequences. Biometrics. 1987 Jun;43(2):261–276. [PubMed] [Google Scholar]
- Bulmer M. Estimating the variability of substitution rates. Genetics. 1989 Nov;123(3):615–619. doi: 10.1093/genetics/123.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBry R. W. The consistency of several phylogeny-inference methods under varying evolutionary rates. Mol Biol Evol. 1992 May;9(3):537–551. doi: 10.1093/oxfordjournals.molbev.a040740. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Phylogenies from molecular sequences: inference and reliability. Annu Rev Genet. 1988;22:521–565. doi: 10.1146/annurev.ge.22.120188.002513. [DOI] [PubMed] [Google Scholar]
- Gu X., Fu Y. X., Li W. H. Maximum likelihood estimation of the heterogeneity of substitution rate among nucleotide sites. Mol Biol Evol. 1995 Jul;12(4):546–557. doi: 10.1093/oxfordjournals.molbev.a040235. [DOI] [PubMed] [Google Scholar]
- Hasegawa M., Kishino H., Yano T. Dating of the human-ape splitting by a molecular clock of mitochondrial DNA. J Mol Evol. 1985;22(2):160–174. doi: 10.1007/BF02101694. [DOI] [PubMed] [Google Scholar]
- Kelly C. A test of the Markovian model of DNA evolution. Biometrics. 1994 Sep;50(3):653–664. [PubMed] [Google Scholar]
- Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980 Dec;16(2):111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- Lake J. A. Reconstructing evolutionary trees from DNA and protein sequences: paralinear distances. Proc Natl Acad Sci U S A. 1994 Feb 15;91(4):1455–1459. doi: 10.1073/pnas.91.4.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanave C., Preparata G., Saccone C., Serio G. A new method for calculating evolutionary substitution rates. J Mol Evol. 1984;20(1):86–93. doi: 10.1007/BF02101990. [DOI] [PubMed] [Google Scholar]
- Nei M., Stephens J. C., Saitou N. Methods for computing the standard errors of branching points in an evolutionary tree and their application to molecular data from humans and apes. Mol Biol Evol. 1985 Jan;2(1):66–85. doi: 10.1093/oxfordjournals.molbev.a040333. [DOI] [PubMed] [Google Scholar]
- Rodríguez F., Oliver J. L., Marín A., Medina J. R. The general stochastic model of nucleotide substitution. J Theor Biol. 1990 Feb 22;142(4):485–501. doi: 10.1016/s0022-5193(05)80104-3. [DOI] [PubMed] [Google Scholar]
- Tajima F., Nei M. Estimation of evolutionary distance between nucleotide sequences. Mol Biol Evol. 1984 Apr;1(3):269–285. doi: 10.1093/oxfordjournals.molbev.a040317. [DOI] [PubMed] [Google Scholar]
- Tamura K., Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol. 1993 May;10(3):512–526. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- Uzzell T., Corbin K. W. Fitting discrete probability distributions to evolutionary events. Science. 1971 Jun 11;172(3988):1089–1096. doi: 10.1126/science.172.3988.1089. [DOI] [PubMed] [Google Scholar]
- Wu C. I., Li W. H. Evidence for higher rates of nucleotide substitution in rodents than in man. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1741–1745. doi: 10.1073/pnas.82.6.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z. Estimating the pattern of nucleotide substitution. J Mol Evol. 1994 Jul;39(1):105–111. doi: 10.1007/BF00178256. [DOI] [PubMed] [Google Scholar]
- Yang Z. Maximum-likelihood estimation of phylogeny from DNA sequences when substitution rates differ over sites. Mol Biol Evol. 1993 Nov;10(6):1396–1401. doi: 10.1093/oxfordjournals.molbev.a040082. [DOI] [PubMed] [Google Scholar]
- Zharkikh A. Estimation of evolutionary distances between nucleotide sequences. J Mol Evol. 1994 Sep;39(3):315–329. doi: 10.1007/BF00160155. [DOI] [PubMed] [Google Scholar]


