Abstract
Background: P2X4 receptor (P2X4R), a purinoceptor expressed in activated spinal microglia, plays a key role in the pathogenesis of neuropathic pain. Spinal nerve injury induces up-regulation of P2X4R on activated microglia in the spinal cord, and blockade of this receptor can reduce neuropathic pain. The present study was undertaken to determine whether paroxetine, an inhibitor of P2X4R, could attenuate allodynia and hyperalgesia in chronic constriction injury (CCI) model of neuropathic pain when used preemptively or after the sciatic nerve injury. Methods: Male Wistar rats (150-200 g, n = 6) were divided into 3 different groups: 1- CCI vehicle-treated group, 2- Sham group, and 3- CCI paroxetine-treated group. Paroxetine (10 mg/kg, i.p.) was administered 1 h before surgery and continued daily until day 14. In other part of the study, paroxetine (10 mg/kg, i.p.) was administered at day 7 post injury and continued daily until day 14. von Frey filaments for mechanical allodynia and analgesia meter for thermal hyperalgesia were used to assay pain behavior. Results: In a preventive paradigm, paroxetine significantly attenuated both mechanical allodynia and thermal hyperalgesia (P<0.001). A significant decrease in pain behavior was seen with paroxetine on existing allodynia (P<0.001) and hyperalgesia (P<0.01) when initiated at day 7 post injury. Conclusion: It seems that paroxetine can attenuate pain behavior when administered before and also after sciatic nerve injury in CCI model of neuropathic pain.
Key Words: Paroxetine, P2X4 receptor (P2X4R), Allodynia, Hyperalgesia
Introduction
Neuropathic pain, mainly due to metabolic disturbance, cancer, or viral infection is associated with lesion of the central or peripheral nervous system. Sensory deficits, which often manifest as allodynia (pain evoked by normally non-painful stimuli) and hyperalgesia (an increased response to painful stimuli), are key diagnostic criteria for the neuropathic pain in animal and human [1]. The mechanism underlying neuropathic pain is unclear, and it is often resistant to general analgesic, such as non-steroidal anti-inflammatory drugs, opioids. However, some anti-depressants have been most widely used in treating neuropathic pain [2, 3].
Anti-depressants have been used for over 30 years to manage neuropathic pain, and there is now substantial evidence to support the use of anti-depressant drugs in the treatment of several intractable pain states, including chronic headache, low back pain, rheumatoid arthritis, and fibromyalgia [4, 5]. Anti-depressants, which inhibit non-selectively the reuptake of serotonin (5-HT) and noradrenaline, have been most widely used drug in patients with diabetic neuropathy [6, 7] and postherpetic neuralgia [8, 9]. Among the selective serotonin reuptake inhibitors, paroxetine is more effective than fluoxetine and citalopram in reducing pain in these patients [10-12]. Interestingly, it was found that the anti-allodynic effect of paroxetine is independent of 5-HT receptor antagonists and was also observed in neuropathic pain animals in which 5-HT was depleted in the spinal cord. These results suggest that the anti-allodynic effect of paroxetine may be mediated by inhibiting P2X4 receptors (P2X4R) [13]. P2X4, a purinoceptor, belongs to a family of ligand-gated ion channels and are cation-selective channels with almost equal permeability to Na+ and K+ and significant permeability to Ca2+ [14]. Recent evidence indicates that P2X4 might be a potential therapeutic target to treat neuropathic pain. It has been shown that activating P2X4 receptors in activated microglia plays a key role in the pathogenesis of neuropathic pain. Spinal nerve injury induces up-regulation of P2X4R on activated microglia in the spinal cord, and spinal blockade of P2X4R produces significant anti-allodynic effects [15]. Among drug used in neuropathic pain, some of which such as minocycline is effective when only administered before injury [16, 17]. It has also been shown that anti-depressants (amitriptyline, mirtazapine, and citalopram) are not able to attenuate tactile neuropathic pain when used after injury [18]. In this regard, the present study evaluated the effect of paroxetine on development and maintenance of pain in chronic constriction injury (CCI) of the sciatic nerve in rats. We investigated whether chronic administration of paroxetine could attenuate allodynia and hyperalgesia when used pre-emptively and when the administration of drug started at day 7 after nerve injury.
MATERIALS AND METHODS
Animals. Male Wistar rats (150-200 g, n = 6) were used. The animals were housed with food and water available ad libitum, in a temperature-controlled environment, with a light-dark cycle of 12:12 h. They were allowed to habituate to the housing facilities for at least 1 week prior to surgery or behavioral testing. Behavioral studies were carried out in a quiet room between the hours 8:00 and 12:00 AM. The experiments were performed according to the Ethical Guidelines of the International Association for the Study of Pain [19].
Surgery. CCI model of neuropathic pain was used [20]. The surgical procedure was performed under ketamine anesthesia (60 mg/kg) and xylazine (10 mg/kg). The left sciatic nerve was exposed, and 4 loose chromic gut ligatures were placed around the nerve proximal to the trifurcation. The distance between two adjacent ligatures was 1 mm. The wound was irrigated with normal saline and closed in two layer with 4-0 silk (fascial plane) and surgical skin staples. In sham-operated group, rats underwent surgical procedure except for the ligation. All surgical procedures were carried out under normal sterile conditions and were performed by the same prson.
Drug preparation. Paroxetine hydrochloride (Sigma, U.S.A) was dissolved in 5% DMSO. Ketamine hydrochloride and Xylazine hydrochloride (both from Sigma, USA) were used for anesthesia. All drugs were injected by the i.p. route.
Drug administration. Animals were divided into two main groups: 1) pre-emptive and 2) post-injury group. Each main group was divided into three different subgroups: I) CCI vehicle-treated group, II) sham group, and III) CCI paroxetine-treated group. Vehicle was injected i.p. to CCI and sham-operated animals. In the pre-emptive study, paroxetine (10 mg/kg) [21] was injected 1 h before surgery and continued daily until day 14 post surgery. In the post-injury group, paroxetine (10 mg/kg) was administered at day 7 post injury and continued daily until day 14. All behavioral tests were recorded on day 0 (control day) before surgery and on days 1, 3, 5, 7, 10, and 14 post-nerve injury.
Behavioral tests and experimental design. The sciatic nerve territory (mid-plantar hind paw) was tested for sensitivity to noxious and innocuous stimuli at several intervals following surgery up to 14 days using standard behavioral assays done sequentially. Animals were acclimated to the testing chambers for 30 min prior to testing. Hyperalgesia and allodynia were evaluated in animals. The order of behavioral tests was therefore defined as follows: thermal hyperalgesia and mechanical allodynia. Animals were left for 30 min undisturbed between each assay.
Thermal hyperalgesia. Thermal hyperalgesia was assessed by means of Hargreaves test [22]. The plantar surface of the paw was exposed to a beam of radiant heat through a transparent perspex surface (Ugo Basile, Comerio, Italy). The withdrawal latency was recorded with a cut-off time of 20 s to prevent tissue damage. The heat stimulation was repeated 5 times at 5-min intervals, and the average value of the withdrawal latency of five consecutive tests was recorded.
Mechanical allodynia. Mechanical sensitivity to non-noxious stimuli was measured by applying a set of calibrated nylon monofilaments (Stoelting, USA). The von Frey methodology was used to assess the sensitivity of the skin to tactile stimulation [23]. Von Frey filaments were calibrated to have a characteristic bending force when pressure was applied. Each rat was placed under a transparent plexiglass cage on an elevated metal screen surface with 1 cm mesh openings. Increasing strengths of von Frey filaments were applied sequentially to the plantar surface of the left hind paw of each animal. The minimum paw withdrawal threshold, defined as the minimum gram strength eliciting two sequential responses with 3-min intervals between them (withdrawal from pressure), was recorded for the left paw. The intensity of mechanical stimulation was increased from 2 to 60 g in a graded manner using successively greater diameter filaments until the hind paw was withdrawn. A paw withdrawal threshold decrease indicates that allodynia has developed. For successive tests, the placement of these stimuli was varied slightly from one trial to the next to avoid sensitization of the hind paw.
Statistical analysis. Parametric data were analyzed for significance with analysis of variance (ANOVA), followed by post-hoc Tukey's test. Non-parametric data were analyzed with related samples followed by the Wilcoxon test. In all cases, data were shown as the mean ± SEM (standard error) and P<0.05 was considered significant.
Results
All animals experienced normal weight gain over the course of the study. Thermal and mechanical stimuli were used over a 14-day time frame.
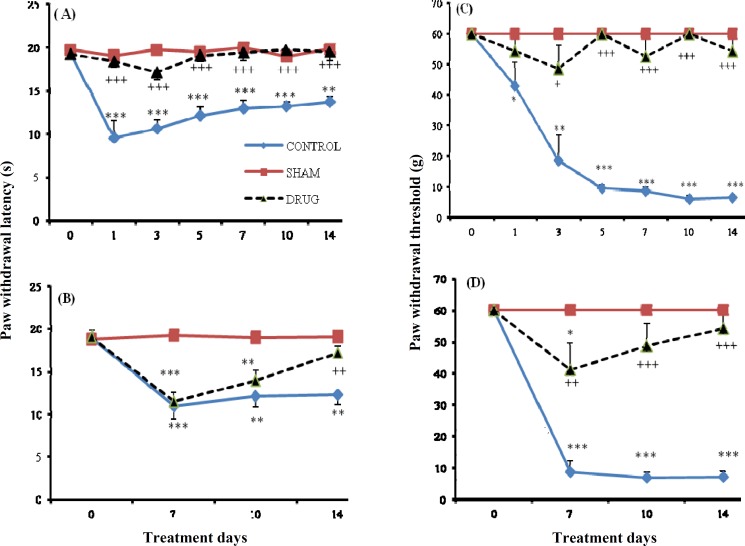
Response to thermal hyperalgesia. In the pre-emptive study, sham-treated rats did not exhibit pain behavior during the 14 days of the study compared to the control day. CCI vehicle-treated group showed thermal hyperalgesia (P<0.001) at the first day following CCI, which was sustained throughout the experimental period. In the CCI paroxetine-treated group, paroxetine (10 mg/kg) attenuated thermal hyperalgesia during the period of study when compared to day 0. In comparison to CCI vehicle-treated group, paroxetine produced a significant decrease in pain behavior (P<0.001) (Fig. 1A). In the post-injury study, CCI vehicle-treated group exhibited pain behavior during the experimental period (P<0.01) compared to day 0. Sham-operated animals did not show thermal hyperalgesia as compared to pre-surgery day. In CCI paroxetine-treated group, paroxetine (10 mg/kg) produced hyperalgesia at days 7 and 10 (P<0.01), but a decrease in pain behavior was seen at day 14 (Fig. 1B).
Fig. 1.
The paw withdrawal latency and threshold in different treatment days. The latency of paw withdrawal (in second) in response to beam of radiant heat before and at several time points after injury (A) and three time points after surgery (B) in CCI vehicle-treated, sham operated and CCI paroxetine-treated group. Paw withdrawal threshold in response to Von Frey filaments before and several time points after injury (C) and three time points after surgery (D) in CCI vehicle-treated group, sham group and CCI paroxetine-group. Paroxetine (10 mg/kg, I.P.) was injected. Data are presented as means ± S.E.M. of 6 rats in each group. Asterisks (*P<0.05, **P<0.01, and ***P<0.001) indicate a statistically significant difference when compared to day 0, and (+P<0.05, ++P<0.001, and +++P<0.001) when compared to CCI vehicle-treated group
Response to mechanical allodynia. In the pre-emptive study, all CCI vehicle-treated animals were strongly allodynic at the first day (post ligation) (P<0.05) compared to control day. This effect was sustained until the end of the study (P<0.001). On the contrary, sham-operated animals did not produce mechanical allodynia throughout the experimental period as compared to pre-surgery day. This effect was also seen in CCI paroxetine-treated group. Moreover, paroxetine (10 mg/kg) significantly attenuated tactile hypersensitivity when compared to CCI vehicle-treated group (P<0.001) (Fig. 1C). In the post-injury study, sham-treated rats did not exhibit pain behavior compared to control day. In the CCI vehicle-treated group, a significant difference in pain behavior (P<0.001) was seen at day 7 post injury compared to day 0. This effect was sustained until the end of study. In the CCI paroxetine-treated group, paroxetine (10 mg/kg) produced pain at day 7 (P<0.05) but not at days 10 and 14 compared to pre-surgery day. An anti-allodynic effect was also produced compared to the CCI vehicle-treated group during the study (P<0.001) (Fig. 1D).
Discussion
In the present study, we evaluated the analgesic effects of paroxetine, an inhibitor of P2X4R in CCI model of neuropathic pain in rat. The analgesic effects of paroxetine were assessed in two ways: pre-emptive and post-injury studies. To date, research using models in rat has mainly focused on injury to a peripheral nerve, usually the sciatic or spinal nerve, to reliably produce behaviors sugges-tive of neuropathic pain in humans [20].
In our study, we found that paroxetine attenuated pain behavior when used before injury and also after inducing an injury to the peripheral nerves. Evidence accumulated from neuropathic pain models suggests that neuropathic pain might involve abnormal excitability of the nervous system. Notably, in primary sensory ganglia and in the dorsal horn of the spinal cord, multiple functional and anatomical alterations of neurons follow peripheral nerve injury [24]. Besides relevant changes in neurons, emerging lines of evidence have revealed that they also occur in glial cells, especially microglia [25].
New roles for nucleotides as neurotransmitters and P2 purinoceptors were recognized [26]. Extracellular ATP is elevated in neuroinflammation in spinal cord injury and neuropathic pain and acts on purinergic receptors such as P2X7 or P2X4 [15]. P2X4 is the most abundant P2X receptor subtype present in the CNS. It is expressed in neurons of different brain regions and in microglia. Increased expression of P2X4 is observed in injured tissue after spinal cord injury, traumatic brain injury, and cerebral ischemia. Moreover, P2X4R are elevated in spinal cord microglia after peripheral nerve injury [27]. Neutral-izing P2X4 activity prevents tactile allodynia, suggesting that P2X4R are involved in neuropathic pain [15].
ATP, acting via P2 purinergic receptors, is a known mediator of inflammatory and neuropathic pain .There is increasing evidence that the ATP-gated P2X4R subtype is a locus through which activity of spinal microglia and peripheral macro-phages instigate pain hypersensitivity caused by inflammation or by injury to a peripheral nerve [28]. There is abundant evidence that P2X4R expression in spinal microglia provides compelling evidence of their functional involvement in tactile allodynia. After peripheral nerve injury, expression of the P2X4R increases in microglia, but not in neurons or astrocytes, in the spinal dorsal horn ipsilateral to the nerve injury. This change in P2X4R expression in microglia can increase the pain hypersensitivity [29]. After peripheral nerve injury, microglia in the spinal cord become activated and show dramatic changes in morphology and robust increases in the expression of microglia markers [30].
Microglia in the dorsal horn of the spinal cord is increasingly recognized as being crucial in the pathogenesis of neuropathic pain after peripheral nerve injury [29]. In this situation, robust microglia cell proliferation occurs after sciatic nerve constriction, partial sciatic nerve ligation, or CCI models of neuropathic pain [30, 31]. Nerve injury results in dramatic up-regulation of the ATP P2X4R in spinal cord microglia [32], indicating that tonic activation of P2X4R in microglia is necessary for sustaining allodynia [15]. Moreover, it has been found that spinal administration of P2X4-stimulated microglia caused otherwise normal rats to develop allodynia. These data suggest that P2X4R activation in microglia is not only necessary but also is sufficient to cause tactile allodynia [26].
It has been shown that activated P2X4R on microglia are also necessary to express and release brain-derived neurotrophic factor by microglia after peripheral nerve injury. Recent findings suggest that the release of brain-derived neurotrophic factor leads to enhancement of neuronal pain transmission [33, 34]. Blocking P2X4R acutely reverse pain behavior after nerve injury, and suppressing this receptor expression attenuates pain hypersensitivity [15]. Therefore, microglia P2X4R are necessary for the ongoing expression of pain behavior after peripheral nerve injury. The above evidence indicates that P2X4 might be a potential therapeutic target to treat neuropathic pain. However, there is no antagonist to strongly inhibit P2X4R [15]. It has recently been shown that some anti-depressants and anti-convulsants used clinically in patients with neuro-pathic pain have inhibitory effects on P2X4R [13]. Among the drugs used, paroxetine has the most potent inhibitory effect on this receptor. Intrathecal administration of paroxetine, but not citalopram, produces an anti-allodynic effect on an animal model of neuropathic pain, which is correlated with the potency of inhibition of rat P2X4R [34]. Moreover, it has been shown that subcutaneous administration of paroxetine can decrease pain behavior in a rat model of neuropathic pain [35]. Another study has demonstrated that i.p. injection of paroxetine reduces mechanical hyperalgesia when used after nerve injury [21]. Reversal of these behavioral hypersensitivities associated with nerve injury by paroxetine is associated with its ability to inhibit P2X4R on the activated microglia at the lumbar spinal cord [13].
Various studies have shown that some drugs are effective in reducing pain behavior in a preventive strategy but not in the existing pain paradigms [16, 17]. It has been shown that acute administration of anti-depressants (Amitriptyline, Mirtazapine, and citalopram) after CCI model of nerve injury in rat is not effective in existing mechanical allodynia [18]. However, administration of duloxetine has been reported to attenuate mechanical allodynia in a rat model of neuropathic pain [36]. On the other hand, other study indicated that after nerve injury, thermal hyperalgesia was completely reversed by amitri-ptyline and duloxetine [18]. It has been found that fluvoxamine produced a much weaker anti-allodynic effect than paroxetine, and as mentioned above, citalopram produced no anti-allodynic effect. However, these selective serotonin reuptake inhibitors (paroxetine, fluvoxamine, and citalopram) have similar inhibitory action on 5-HT transporters. These results indicate that the difference in the potency of inhibition on P2X4R may explain the difference in the clinical effectiveness of anti-depressants in patients with neuropathy [3].
Our data showed that paroxetine reduced pain behavior when administered preventively, which is consistent with the above mentioned studies. On the other hand, we found that paroxetine was able to decrease pain behavior in already existing pain in contrast to other studies in which some drugs had no effect on reduction of pain after injury [16-18].
In conclusion, it seems that paroxetine, an inhibitor of P2X4R on activated microglia, could significantly reverse mechanical allodynia and thermal hyperalgesia when used pre-emptively. In our study, we found that in the post-injury study, paroxetine was also able to attenuate mechanical allodynia during the experimental period, but there was a delay in decreasing thermal hyperalgesia (the effect that was seen at day 14 post surgery). Therefore, both the development and existing pain in a CCI model of neuropathic pain were reduced by paroxetine. According to the diversity of the mechanisms involved in the neuropathic pain, further evaluation is required to understand the exact mechanism of action of paroxetine in this condition.
ACKNOWLEDGMENTS
This research is extracted from Malek Zarei’s Ph.D. dissertation of Pharmacology, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
References
- 1.Saghaei E, Zanjani T, Sabetkasaei M, Naseri K. Enhancement of antinociception by co-administrations of nefopam, morphine, and nimesulide in a rat model of neuropathic pain. Korean J Pain. 2012 Jan;25(1):7–15. doi: 10.3344/kjp.2012.25.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang X, XU Y, Wang J, Zhou Q, Pu S, Jiang W, et al. The effect of intrathecal administration of glial activation inhibitors on dorsal horn BDNF overexpression and hind paw mechanical allodynia in spinal nerve ligated rats. J Neural Transm. 2012 Mar;119(3):329–36. doi: 10.1007/s00702-011-0713-7. [DOI] [PubMed] [Google Scholar]
- 3.Wang W, Gu J, Li YQ, Tao YX. Are voltage-gated sodium channels on the dorsal root ganglion involved in the development of neuropathic pain? Mol Pain. 2011 Feb;7:16. doi: 10.1186/1744-8069-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sindrup SH, Otto M, Finnerup NB, Jensen TS. Antidepressants in the treatment of neuropathic pain. Basic Clin Pharmacol Toxicol. 2005 Jun;96(6):399–409. doi: 10.1111/j.1742-7843.2005.pto_96696601.x. [DOI] [PubMed] [Google Scholar]
- 5.Lynch ME. Antidepressants as analgesics: a review of randomized controlled trials. J Psychiatry Neurosci. 2001 Jan;26(1):30–6. [PMC free article] [PubMed] [Google Scholar]
- 6.Max MB, Kishore-Kumar R, Schafer SC, Meister B, Gracely RH, Smoller B, et al. Efficacy of desipramine in painful diabetic neuropathy: a placebo-controlled trial. Pain. 1991 Apr;45(1):3–9. doi: 10.1016/0304-3959(91)90157-S. [DOI] [PubMed] [Google Scholar]
- 7.Sindrup SH, Tuxen C, Gram LF, Grodum E, Skjold T, Brosen K, et al. Lack of effect of mianserin on the symptoms of diabetic neuropathy. Eur J Clin Pharmacol. 1992;43(3):251–5. doi: 10.1007/BF02333018. [DOI] [PubMed] [Google Scholar]
- 8.Kishore-Kumar R, Max MB, Schafer SC, Gaughan AM, Smoller B, Gracely RH, et al. Desipramine relieves postherpetic neuralgia. Clin Pharmacol Ther. 1990 Mar;47(3):305–12. doi: 10.1038/clpt.1990.33. [DOI] [PubMed] [Google Scholar]
- 9.Raja SN, Haythornthwaite JA, Pappagallo M, Clark MR, Travison TG, Sabeen S, et al. Opioids versus antidepressants in postherpetic neuralgia: a randomized, placebo-controlled trial. Neurology. 2002 Oct;59:1015–21. doi: 10.1212/wnl.59.7.1015. [DOI] [PubMed] [Google Scholar]
- 10.Max MB, Lynch SA, Muir J, Shoaf SE, Smoller B, Dubner R. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med. 1992 May;326(19):1250–6. doi: 10.1056/NEJM199205073261904. [DOI] [PubMed] [Google Scholar]
- 11.Jackson KC. Pharmacotherapy for neuropathic pain. Pain Pract. 2006 Mar;6(1):27–33. doi: 10.1111/j.1533-2500.2006.00055.x. [DOI] [PubMed] [Google Scholar]
- 12.Sindrup SH, Gram LF, Brosen K, Eshoj O, Mogensen EF. The selective serotonin reuptake inhibitor paroxetine is effective in the treatment of diabetic neuropathy symptoms. Pain. 1990 Aug;42:135–44. doi: 10.1016/0304-3959(90)91157-E. [DOI] [PubMed] [Google Scholar]
- 13.Nagata K, Imai T, Yamashita T, Tsuda M, Tozaki-Saitoh H, Inoue K. Antidepressants inhibit P2X4 receptor function: a possible involvement in neuropathic pain relief. Mol Pain. 2009 Apr;5:20. doi: 10.1186/1744-8069-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002 Oct;82(4):1013–67. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 15.Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, et al. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003 Aug;424(6950):778–83. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- 16.Padi SS, Kulkarni SK. Minocycline prevents the development of neuropathic pain, but not acute pain: Possible anti-inflammatory and antioxidant mechanisms. Euro J Pharmacol. 2008 Dec;601(1-3):79–87. doi: 10.1016/j.ejphar.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 17.Raghavendra V, Tanga F, Deleo J. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther. 2003 Aug;306(2):624–30. doi: 10.1124/jpet.103.052407. [DOI] [PubMed] [Google Scholar]
- 18.Bomholt SF, Mikkelsen JD, Blackburn-Munro G. Antinociceptive effects of the antidepressants amitriptyline, duloxetine, mirtazapine and citalopram in animal models of acute, persistent and neuropathic pain. Neuropharmacology. 2005 Feb;48(2):252–63. doi: 10.1016/j.neuropharm.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983 Jun;16(2):109–10. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 20.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in men. Pain. 1988 Apr;33(1):87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 21.Nakjima K, Obata H, Iriuchijima N, Saito S. An increase in spinal cord noradrenaline is a major contributor to the antihyperalgesic effect of antidepressant after peripheral nerve injury in the rat. Pain. 2012 May;153(5):990–7. doi: 10.1016/j.pain.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 22.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988 Jan;32(1):77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 23.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994 Jul;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 24.Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10:1361–8. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- 26.Inoue K, Tsuda M. Purinergic systems, neuropathic pain and the role of microglia. Exp Neurol. 2012 Apr;234(2):293–301. doi: 10.1016/j.expneurol.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 27.de Rivero Vaccari JP, Bastien D, Yurcisin G, Pineau I, Dietrich WD, et al. P2X4 receptors influence inflammasome activation after spinal cord injury. J Neurosci. 2012 Feb;32(9):3058–66. doi: 10.1523/JNEUROSCI.4930-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trang T, Salter MW. P2X4 purinoceptor signaling in chronic pain. Purinergic Signal. 2012 Sep;8(3):621–8. doi: 10.1007/s11302-012-9306-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiaodi Y, Shuangqiong , Qianbo Ch, Chengwen CH, Hongbin Y. P2X4 receptor and brain-derived neurotrophic factor in neuropathic pain. J Med Coll PLA. 2010;25:275–284. [Google Scholar]
- 30.Wen YR, Tan PH, Cheng JK, Liu YC, Ji RR. Microglia: a promising target for treating neuropathic and pain, and morphine tolerance. J Formos Med Assoc. 2011 Aug;110(8):487–94. doi: 10.1016/S0929-6646(11)60074-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Echeverry S, Shi XQ, Zhang J. Characterization of cell proliferation in rat spinal cord following peripheral nerve injury and the relationship with neuropathic pain. Pain. 2008 Mar;135(1-2):37–47. doi: 10.1016/j.pain.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Zhuang ZY, Kawasaki Y, Tan PH, Wen YR, Huang J, Ji RR. Role of the CX3CR1/p38 MAPK pathway in spinal microglia for the development of neuropathic pain following nerve injury induced cleavage of fractalkine. Brain Behav Immun. 2007 Jul;21(5):642–51. doi: 10.1016/j.bbi.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trang T, Beggs S, Wan X, Salter MW. P2X4-receptor-mediated synthesis and release of brain-derived neurotrophic factor in microglia is dependent on calcium and p38-mitogen-activated protein kinase activation. J Neurosci. 2009 Mar;29(11):3518–28. doi: 10.1523/JNEUROSCI.5714-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lever I, Cunningham J, Grist J, Yip PK, Malcangio M. Release of BDNF and GABA in the dorsal horn of neuropathic rats. Eur J Neurosci. 2003 Sep;18(5):1169–74. doi: 10.1046/j.1460-9568.2003.02848.x. [DOI] [PubMed] [Google Scholar]
- 35.Leventhal L, Smith V, Hornby G, Andree TH, Brandt MR, Rogres KE. Differential and synergistic effects of selective norepinephrine and serotonin reuptake inhibitors in rodent models of pain. J Pharmacol Exp Ther. 2007 Mar;320(3):1178–85. doi: 10.1124/jpet.106.109728. [DOI] [PubMed] [Google Scholar]
- 36.Iyengar S, Webster AA, Hemrick-Luecke SK, Xu JY, Simmons RM. Efficacy of duloxetine, a potent and balanced serotonin-norepinephrine reuptake inhibitor in persistent pain models in rats. J Pharmacol Exp Ther. 2004 Nov;311(2):576–84. doi: 10.1124/jpet.104.070656. [DOI] [PubMed] [Google Scholar]