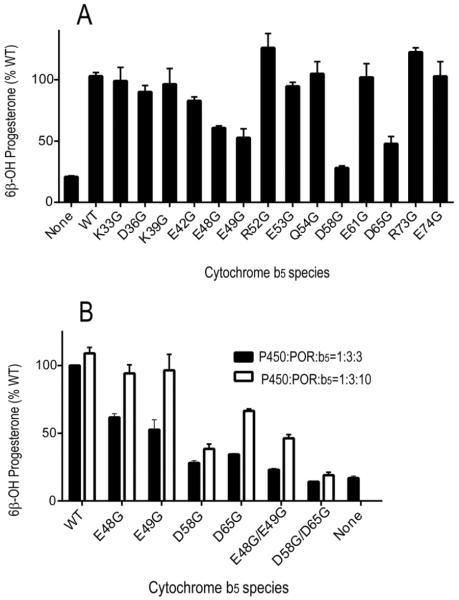
FIGURE 1.
Stimulation of CYP3A4-catalyzed progesterone 6β-hydroxylation by b5 and b5 mutations. (A) Effect of b5 and b5 mutations on catalytic activities of CYP3A4 in the presence of CYMS as lipid for reconstitution. Incubations contained 50 μM progesterone and a molar ratio of P450:POR:b5 at 1:3:3. Results are shown as the percentage activity compared to wild-type b5 values (=100%) from triplicate determinations, means ± standard deviations. (B) Comparison of wild-type b5 and selected b5 mutations on CYP3A4 catalysis with molar ratio of P450:POR:b5 at 1:3:3 (black bars) or 1:3:10 (white bars). Results are shown as the percentage activity compared to wild-type b5 values with 1:3:3 ratio of P450:POR:b5 (=100%) from triplicate determinations, means ± standard deviations.