Abstract
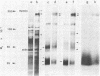
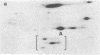
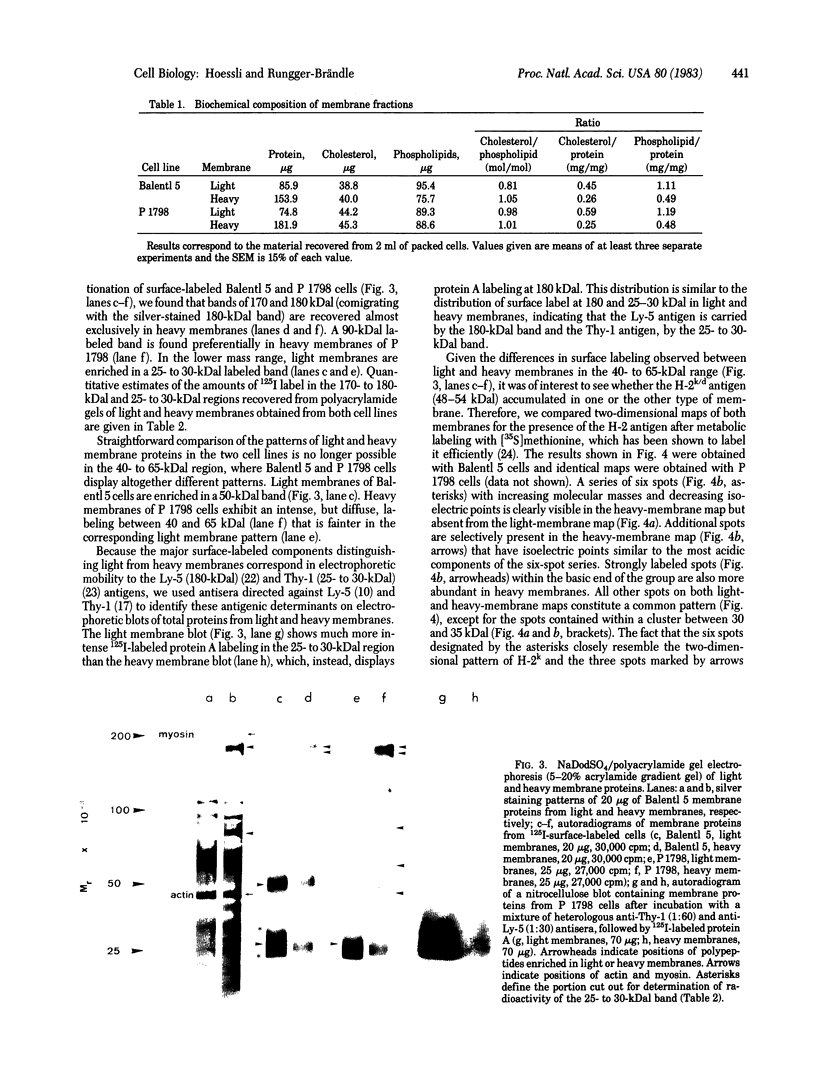
Murine T-lymphoma cells have been homogenized in dense sucrose solution and centrifuged under isopycnic conditions for membrane components. Floating fractions banding between 10% and 22.5% sucrose ("light" membranes) and between 22.5% and 35% sucrose ("heavy" membranes) were shown to consist of smooth membrane vesicles. The amounts of cholesterol and phospholipids recovered after chloroform/methanol extraction were similar in both fractions, but heavy membranes contained two to three times more protein than light membranes. The most striking difference between the two membrane fractions was revealed by their labeled surface glycoprotein patterns on polyacrylamide gels, suggesting that (i) the smooth membrane vesicles originated from the plasma membrane and (ii) two distinct segments of the plasma membrane can be recovered in fractions characterized by specific surface glycoproteins. Light membranes were enriched in Thy-1 antigen, whereas Ly-5 antigen and a 170,000-dalton surface glycoprotein were recovered almost exclusively from heavy membranes, as were metabolically labeled protein spots comigrating with the H-2k/d antigen in two-dimensional electrophoresis. The patterns of the unlabeled proteins in light and heavy membranes appeared similar, except for polypeptides of 180,000 and 85,000 daltons that were found preferentially in heavy membranes. These results support the concept of plasma membrane domains by showing that two distinct populations of plasma membrane vesicles can be isolated and that these populations contain different sets of cell surface glycoproteins.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Crawford L. V., Gesteland R. F. Synthesis of polyoma proteins in vitro. J Mol Biol. 1973 Mar 15;74(4):627–634. doi: 10.1016/0022-2836(73)90053-3. [DOI] [PubMed] [Google Scholar]
- Demus H. Subcellular fractionation of human lymphocytes. Isolation of two plasma membrane fractions and comparison of the protein components of the various lymphocytic organelles. Biochim Biophys Acta. 1973 Jan 2;291(1):93–106. doi: 10.1016/0005-2736(73)90064-3. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Fechheimer M., Cebra J. J. Isolation and characterization of actin and myosin from B-lymphocytic guinea pig leukemia cells. J Immunol. 1979 Jun;122(6):2590–2597. [PubMed] [Google Scholar]
- Hoessli D. C., Vassalli P. High molecular weight surface glycoproteins of murine lymphocytes. J Immunol. 1980 Oct;125(4):1758–1763. [PubMed] [Google Scholar]
- Hoessli D. C., Vassalli P., Pink J. R. Characterization of mouse thymocyte and peripheral lymphocyte xenoantigens by two-dimensional electrophoresis. Eur J Immunol. 1980 Nov;10(11):814–821. doi: 10.1002/eji.1830101104. [DOI] [PubMed] [Google Scholar]
- Hoessli D., Bron C., Pink J. R. T-lymphocyte differentiation is accompanied by increase in sialic acid content of Thy-1 antigen. Nature. 1980 Feb 7;283(5747):576–578. doi: 10.1038/283576a0. [DOI] [PubMed] [Google Scholar]
- Jett M., Seed T. M., Jamieson G. A. Isolation and characterization of plasma membranes and intact nuclei from lymphoid cells. J Biol Chem. 1977 Mar 25;252(6):2134–2142. [PubMed] [Google Scholar]
- Klausner R. D., Kleinfeld A. M., Hoover R. L., Karnovsky M. J. Lipid domains in membranes. Evidence derived from structural perturbations induced by free fatty acids and lifetime heterogeneity analysis. J Biol Chem. 1980 Feb 25;255(4):1286–1295. [PubMed] [Google Scholar]
- Klausner R. D., Kumar N., Weinstein J. N., Blumenthal R., Flavin M. Interaction of tubulin with phospholipid vesicles. I. Association with vesicles at the phase transition. J Biol Chem. 1981 Jun 10;256(11):5879–5885. [PubMed] [Google Scholar]
- Klingenberg M. Membrane protein oligomeric structure and transport function. Nature. 1981 Apr 9;290(5806):449–454. doi: 10.1038/290449a0. [DOI] [PubMed] [Google Scholar]
- Loor F. Plasma membrane and cell cortex interactions in lymphocyte functions. Adv Immunol. 1980;30:1–120. doi: 10.1016/s0065-2776(08)60194-7. [DOI] [PubMed] [Google Scholar]
- Mathieson B. J., Campbell P. S., Potter M., Asofsky R. Expression of Ly 1, Ly 2, Thy 1, and TL differentiation antigens on mouse T-cell tumors. J Exp Med. 1978 Apr 1;147(4):1267–1279. doi: 10.1084/jem.147.4.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher M. F., Jose M. J., Balk S. P. Actin-containing matrix associated with the plasma membrane of murine tumour and lymphoid cells. Nature. 1981 Jan 15;289(5794):139–144. doi: 10.1038/289139a0. [DOI] [PubMed] [Google Scholar]
- Monneron A., d'Alayer J. Isolation of plasma and nuclear membranes of thymocytes. I. Enzymatic composition and ultrastructure. J Cell Biol. 1978 Apr;77(1):211–231. doi: 10.1083/jcb.77.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monneron A., d'Alayer J. Isolation of plasma and nuclear membranes of thymocytes. II. Biochemical composition. J Cell Biol. 1978 Apr;77(1):232–245. doi: 10.1083/jcb.77.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore N. F., Patzer E. J., Barenholz Y., Wagner R. R. Effect of phospholipase C and cholesterol oxidase on membrane integrity, microviscosity, and infectivity of vesicular stomatitis virus. Biochemistry. 1977 Oct 18;16(21):4708–4715. doi: 10.1021/bi00640a027. [DOI] [PubMed] [Google Scholar]
- Nathenson S. G., Uehara H., Ewenstein B. M., Kindt T. J., Coligan J. E. Primary structural: analysis of the transplantation antigens of the murine H-2 major histocompatibility complex. Annu Rev Biochem. 1981;50:1025–1052. doi: 10.1146/annurev.bi.50.070181.005113. [DOI] [PubMed] [Google Scholar]
- Omary M. B., Trowbridge I. S. Disposition of T200 glycoprotein in the plasma membrane of a murine lymphoma cell line. J Biol Chem. 1980 Feb 25;255(4):1662–1669. [PubMed] [Google Scholar]
- Omary M. B., Trowbridge I. S., Scheid M. P. T200 cell surface glycoprotein of the mouse. Polymorphism defined by the Ly-5 system of alloantigens. J Exp Med. 1980 May 1;151(5):1311–1316. doi: 10.1084/jem.151.5.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Switzer R. C., 3rd, Merril C. R., Shifrin S. A highly sensitive silver stain for detecting proteins and peptides in polyacrylamide gels. Anal Biochem. 1979 Sep 15;98(1):231–237. doi: 10.1016/0003-2697(79)90732-2. [DOI] [PubMed] [Google Scholar]
- Tartakoff A., Hoessli D., Vassalli P. Intracellular transport of lymphoid surface glycoproteins. Role of the Golgi complex. J Mol Biol. 1981 Aug 25;150(4):525–535. doi: 10.1016/0022-2836(81)90378-8. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S., Mazauskas C. Immunological properties of murine thymus-dependent lymphocyte surface glycoproteins. Eur J Immunol. 1976 Aug;6(8):557–562. doi: 10.1002/eji.1830060806. [DOI] [PubMed] [Google Scholar]
- Williams A. F., Gagnon J. Neuronal cell Thy-1 glycoprotein: homology with immunoglobulin. Science. 1982 May 14;216(4547):696–703. doi: 10.1126/science.6177036. [DOI] [PubMed] [Google Scholar]