Abstract
Objective
To identify new genetic associations with juvenile and adult dermatomyositis (DM).
Methods
We performed a genome-wide association study (GWAS) of adult and juvenile DM patients of European ancestry (n = 1178) and controls (n = 4724). To assess genetic overlap with other autoimmune disorders, we examined whether 141 single nucleotide polymorphisms (SNPs) outside the major histocompatibility complex (MHC) locus, and previously associated with autoimmune diseases, predispose to DM.
Results
Compared to controls, patients with DM had a strong signal in the MHC region consisting of GWAS-level significance (P < 5x10−8) at 80 genotyped SNPs. An analysis of 141 non-MHC SNPs previously associated with autoimmune diseases showed that three SNPs linked with three genes were associated with DM, with a false discovery rate (FDR) < 0.05. These genes were phospholipase C like 1 (PLCL1, rs6738825, FDR=0.00089), B lymphoid tyrosine kinase (BLK, rs2736340, FDR=0.00031), and chemokine (C-C motif) ligand 21 (CCL21, rs951005, FDR=0.0076). None of these genes was previously reported to be associated with DM.
Conclusion
Our findings confirm the MHC as the major genetic region associated with DM and indicate that DM shares non-MHC genetic features with other autoimmune diseases, suggesting the presence of additional novel risk loci. This first identification of autoimmune disease genetic predispositions shared with DM may lead to enhanced understanding of pathogenesis and novel diagnostic and therapeutic approaches.
Keywords: dermatomyositis, adult, juvenile, shared autoimmunity genes
The idiopathic inflammatory myopathies, or myositis syndromes, are a heterogeneous group of systemic disorders that have been proposed to be autoimmune diseases based largely on the presence of unique autoantibodies and/or self-directed T or B lymphocyte responses in some subsets of patients [1]. Myositis patients themselves can develop additional autoimmune diseases, and there is an elevated occurrence of other autoimmune diseases in close relatives [2; 3]. Recent genome-wide association studies (GWAS) have identified many novel genes associated with several autoimmune diseases [4]. However, outside of the human leukocyte antigen region, there is limited direct evidence supporting a genetic relationship between the idiopathic inflammatory myopathies and other autoimmune disorders [5]. The idiopathic inflammatory myopathies are relatively rare, with a prevalence of 10–15 cases per 100,000, and this has hindered progress in genetic mapping studies [6].
We assembled a large international collection of samples from subjects with dermatomyositis (DM), the most frequent and readily identified phenotype of the idiopathic inflammatory myopathies, to identify new genetic associations with myositis. DM is defined by pathognomonic rashes and chronic muscle inflammation, consisting primarily of CD4+ T lymphocytes, B lymphocytes, dendritic cells, and macrophages [1; 7]. DM in adults and children has similar clinical and pathologic features [6; 8] that likely share pathogenic mechanisms, including the involvement of type I interferon pathways [7]. To define the genetic architecture of DM, we performed the first GWAS of this disease, which confirmed a strong signal in the major histocompatibility complex (MHC) region and revealed enrichment of genetic loci that have been associated with a variety of other autoimmune disorders.
PATIENTS AND METHODS
Study populations
Investigators with collections of DNA samples from myositis patients formed a collaboration called the Myositis Genetics Consortium (MYOGEN) with the goal of identifying new genetic factors associated with myositis. We focused our first study on DM because of its relatively higher frequency in children and adults and more homogeneous features compared to other myositis phenotypes [6]. The criteria for inclusion of DM cases were predetermined to be probable or definite DM as defined by proximal weakness, myopathy on electromyography, muscle biopsy consistent with idiopathic inflammatory myopathy or elevated serum muscle enzymes, and the presence of Gottron’s papules/sign or heliotrope rash, with exclusion of other causes of muscle disease per Bohan and Peter criteria [9]. Age at onset of less than 18 years defined juvenile DM. After excluding 241 cases due to low call rates (n = 123), outliers (n = 55), or related individuals (n = 48), 1178 Caucasian cases with either adult DM (n = 705) or juvenile DM (n = 473) from clinical centers in the US and Europe were analyzed.
The US cases were obtained from three centers, including the National Institutes of Health (234 adult DM and 140 juvenile DM), the Mayo Clinic (53 adult DM and 36 juvenile DM), and the Children’s Memorial Research Center in Chicago (107 juvenile DM). The UK cases were obtained from the UK Adult Onset Myositis Immunogenetic Collaboration (149 adult DM) and the UK Juvenile Dermatomyositis Research Group (159 cases). Other European samples came from the Czech Republic (114 adult and 11 juvenile DM), Hungary (64 adult and 12 juvenile DM), Spain (43 adult and 4 juvenile DM), Sweden (37 adult and 4 juvenile DM), and the Netherlands (11 adult DM).
In order to optimize case-control matching, we utilized separate control groups for each geographic collection of patients. For control samples, single nucleotide polymorphism (SNP) genotyping of healthy Czech and Hungarian volunteers from the Institute of Rheumatology, Prague, Czech Republic or the University of Debrecen, Debrecen, Hungary was performed on either the Illumina Human1M-Duo v3 BeadChip (n = 235: 166 Czechs and 69 Hungarians) or the Illumina Human660W-Quad v1 BeadChip (n = 21: all Hungarian). US controls were taken from previously available data from the North American Rheumatoid Arthritis Consortium [10]. UK controls were taken from the available data from The Wellcome Trust Case-Control Consortium (WTCCC 1958 birth cohort on the Illumina Human1M-Duo v3 BeadChip (n = 2415; http://www.wtccc.org.uk/ccc1). Swedish and Dutch controls (n = 642) were taken from previously published datasets [11], and Spanish controls (n = 259) were obtained from blood bank volunteers in Granada, Spain using data generated on the Illumina Human1M-Duo v3 BeadChip. All subjects consented to be enrolled in protocols approved by local ethics boards.
Genotyping and quality control
Genotyping of cases was carried out using various Illumina GWAS arrays at the Feinstein Institute for Medical Research, Manhasset, New York, US. Since the genotyping was done over several years, the specific Illumina chip used for analysis was upgraded as new platforms became available. Among the cases, 86 were genotyped using the Illumina HumanHap550 BeadChip, 221 were genotyped using the Illumina HumanCNV370-Duo v1 BeadChip, 293 were genotyped using the Illumina Human610-Quad v1 BeadChip, and 578 were genotyped using the Illumina Human660W-Quad v1 BeadChip, according to the manufacturer’s protocols (Illumina Inc., San Diego, CA). Only SNPs that were present on all platforms were evaluated. SNPs that yielded P < 0.001 in association tests between cases genotyped on different chips within each geographic group were dropped in the final results (n=1372).
All data underwent quality control before merging and final statistical analyses. The following data were excluded: SNPs with a call rate of <95% on any platform, individuals with >10% missing rates in genotypes, and SNPs of minor allele frequency of ≤0.01 or Hardy-Weinberg equilibrium in controls with a P value ≤ 10−5. Merged data were separated into five groups according to geographic region. Relatedness was checked by estimating the identity-by-descent coefficient in PLINK (http://pngu.mgh.harvard.edu/purcell/plink/) [12].
A PI-HAT (representing the estimated identity-by-descent sharing among relatives, with 0 indicating unrelated and 1 indicating an identical twin) threshold > 0.15 was used, and we retained only one member of each set of duplicated or related samples (n=48). Outliers identified in the clustering in PLINK (Z > 4 or < −4) were removed (n=15). Additional outliers (n=6) that deviated by more than 4 standard errors from the centroid were identified by principal component analysis in Eigenstrat (http://genepath.med.harvard.edu/~reich/Software.htm) using 16,819 SNPs that are in the linkage disequilibrium (LD)-pruned SNP set provided by The Gene, Environment Association Studies consortium (GENEVA) coordinating center [13]. We included the principal components in which cases and controls had significantly different loadings for each site, and this analysis required that we adjust for the top five principal components for analysis of the US data, no principal components for analysis of the UK data, six principal components for analysis of the Dutch data, one principal component for analysis of Central European data, and one principal component for analysis of Spanish data.
Statistical analysis
The additive model was used in the PLINK logistic association test for each group separately, including the top principal components as covariates to remove residual population structure. Then meta-analysis using PLINK was done for all five groups. For the focused analysis of autoimmune-related SNPs, we adopted a Benjamini Hochberg false discovery rate (FDR) of <0.05.
RESULTS
GWAS identified the MHC locus as the strongest genetic risk region for DM
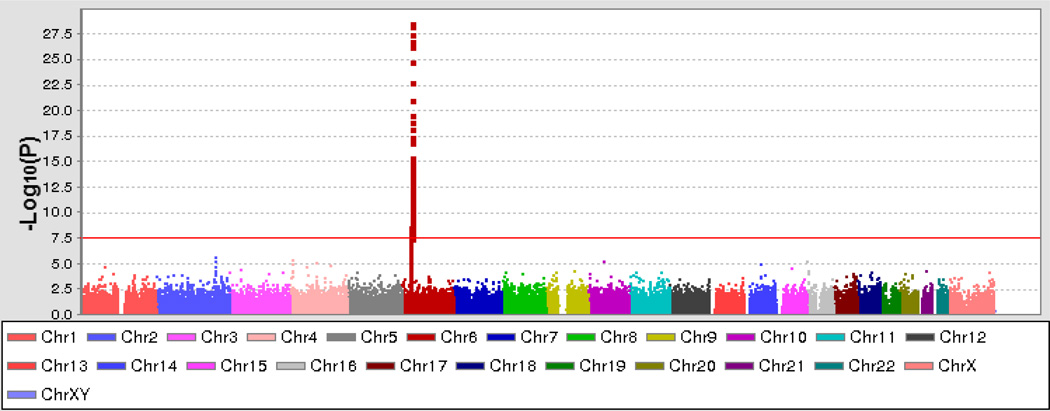
The GWAS of 1178 cases and 4724 control samples included in this study (Table 1) showed GWAS-level significance (P < 5x10−8) at 80 genotyped SNPs across the MHC region (Figure 1), which is consistent with prior targeted gene studies that associated this region with myositis phenotypes [5]. No significant differences were noted between males and females or between adult and juvenile DM in these analyses.
Table 1.
Characteristics of the dermatomyositis cases, controls and SNP data included in the study
| Population | Cases |
Controls |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Adult dermatomyositis | Juvenile dermatomyositis |
No. of successfully genotyped SNPs |
Covariate | Genomic inflation factor (λ) | |||||
| Sample size | Female (%) |
Sample size | Female (%) |
Sample size |
Female (%) |
||||
| Czech/Hungarian | 178 | 70.8% | 23 | 78.3% | 256 | 57.4% | 242530 | Population structure | 1.01 |
| Spanish | 43 | 81.4% | 4 | 50.0% | 259 | 65.6% | 242871 | Population structure | 1.009 |
| Swedish/Dutch | 48 | 68.8% | 4 | 75.0% | 642 | 72.4% | 242644 | Population structure | 1.021 |
| UK | 149 | 65.8% | 159 | 70.4% | 2415 | 47.8% | 236039 | None | 1 |
| USA | 287 | 76.0% | 283 | 69.6% | 1152 | 70.8% | 237155 | Population structure | 1.073 |
| Meta-analysis | 705 | 72.4% | 473 | 70.2% | 4724 | 58.2% | 241502 | None | 1.043 |
Figure 1.
Results of genome-wide association analysis of dermatomyositis plotted on a genomic scale (Manhattan plot) showing P values for 242,876 successfully genotyped single-nucleotide polymorphisms. The orange line represents the genome-wide level of significance (P = 5x10−8). Chr = chromosome.
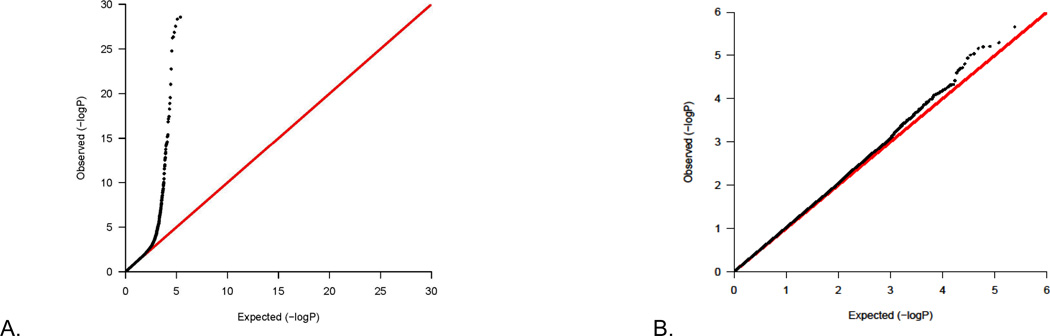
We used quantile-quantile (Q-Q) plots, which is a method for comparing two probability distributions by plotting quantiles against each other, to evaluate the comparability of tests we conducted to their expected distributions. We included any significant principal components as covariates to remove the residual population structure in GWAS for each geographic group before the meta-analysis (Table 1); therefore, we did not adjust population structure again in the meta-analysis. For the fixed-effect P values of genotyped SNPs in the GWAS meta-analysis, when comparing the observed versus the expected distribution of tests, we found no overall systematic inflation of the number of positive tests (Figure 2A), as the ratio of the median chi-square test to the expected value gave a lambda ratio of 1.043, which is close to the expected value of 1.0 (Table 1). These findings were essentially unchanged after eliminating the MHC region (lambda = 1.037, Figure 2B). The random-effect P values of genotyped SNPs in the GWAS meta-analysis were essentially the same as or very similar to the fixed-effect P values (data not shown).
Figure 2.
(A) Quantile-quantile (Q-Q) plot of the genome-wide meta-analysis (lambda = 1.043). (B) Q-Q plot of the genome-wide meta-analysis without the major histocompatibility complex region (lambda = 1.037).
GWAS of DM reveals genetic overlap with other autoimmune disorders
Given the familial aggregation of DM with several common autoimmune diseases, we tested the hypothesis that DM has a genetic architecture similar to that of other autoimmune diseases that have been found to be associated with first-degree relatives of DM patients [2; 3]. Therefore, we selected 269 SNPs that had been associated with rheumatoid arthritis (RA) [14; 15], systemic lupus erythematosus (SLE) [16; 17], type 1 diabetes [18; 19] [20], Crohn’s disease [21; 22] [23], thyroid disease [24], gluten-sensitive enteropathy [25], or multiple sclerosis [26], and assessed their association with DM. Of these 269 SNPs, 141 were genotyped or were in LD (r2 >0.9) with genotyped SNPs in DM, based on publicly accessible LD data from Hapmap 3 CEU (see data in Supplementary Table S1 for all 141 SNPs). Of these 141 SNPs, SNPs related to three genes, which had not been previously associated with DM, were found to have significant (FDR < 0.05) associations with DM (Table 2). These SNPs were related to phospholipase C like 1 (PLCL1: rs6738825 in LD with rs7572733, FDR=0.00089, also in LD with rs1518364, FDR=0.0037, and in LD with rs938929, FDR=0.0030); B lymphoid tyrosine kinase (BLK: rs2736340, FDR=0.0031); and chemokine (C-C motif) ligand 21 (CCL21: rs951005, FDR=0.0076, and in LD with rs2492358, FDR=0.0060) (see data in Supplementary Table S1 for all 141 SNPs). None of these SNPs was in LD with SNPs from the other genes. Minor variations were noted in the SNP associations between the adult and juvenile DM cohorts, but no significant differences were seen.
Table 2.
Overlap of published genome-wide association study single nucleotide polymorphisms for autoimmune diseases with those for dermatomyositis*
| Gene Name |
SNP Marker | Original SNP/LD | Chr: Position | OR (CI) | P | FDR | SNP Disease Source [ref] |
|---|---|---|---|---|---|---|---|
| PLCL1 | rs7572733 | rs6738825/0.979 | 2: 198929806 | 0.80 (0.72–0.88) | 6.18E-06 | 0.00089 | SLE [17] |
| PLCL1 | rs1518364 | rs6738825/0.958 | 2: 198809975 | 1.22 (1.11–1.35) | 5.11E-05 | 0.0037 | SLE [17] |
| PLCL1 | rs938929 | rs6738825/0.958 | 2: 198780860 | 1.22 (1.10–1.34) | 0.00008322 | 0.0030 | SLE [17] |
| BLK | rs2736340 | 8: 11343973 | 1.25 (1.12–1.40) | 0.0000653 | 0.0031 | RA [14] | |
| CCL21 | rs2492358 | rs951005/1.0 | 9: 34737828 | 0.77 (0.67–0.88) | 0.0002093 | 0.0060 | RA [14] |
| CCL21 | rs951005 | 9: 34743681 | 0.77 (0.67–0.89) | 0.000317 | 0.0076 | RA [14] |
Only SNPs with FDR < 0.05 are listed; SNP marker = directly genotyped single nucleotide polymorphism (SNP) by genome-wide association studies; Original SNP = original SNPs among 141 SNPs associated with autoimmune diseases, if not directly genotyped; LD = linkage disequilibrium in r2 with the directly genotyped SNP on Illumina arrays; Chr = chromosome; Position = base pair in hg19/build37 coordinate; OR = odds ratio; CI = 95% confidence interval; P = fixed effect P value in meta-analysis; FDR = false discovery rate; SLE = systemic lupus erythematosus; RA = rheumatoid arthritis.
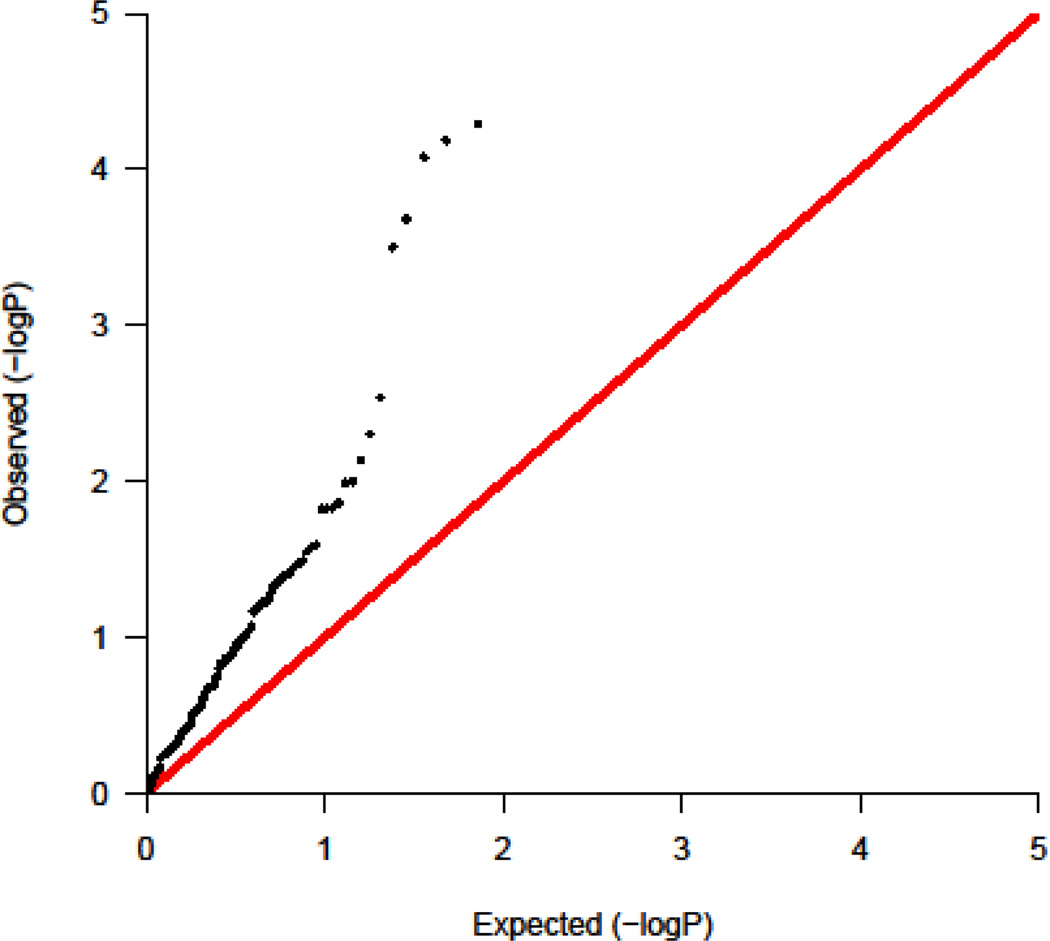
To assess the relevance of these autoimmune-related SNPs to DM, we evaluated Q-Q plots of these SNPs in DM and found a marked excess of positive associations of these SNPs with DM across the range of variants (Figure 3, lambda = 2.59). The current study had a low value of lambda in the entire population of SNPs that had been genotyped.
Figure 3.
Quantile-quantile (Q-Q) plot showing an excess of positive associations of published genome-wide association study non-major histocompatibility complex single-nucleotide polymorphisms for autoimmune diseases with those for dermatomyositis (lambda = 2.59).
DISCUSSION
This work, which to our knowledge is the first GWAS of any form of myositis, is consistent with previous targeted studies suggesting that the MHC is the major genetic region associated with DM [5]. In addition, we have provided initial evidence that a number of non-MHC genes that were previously associated with other autoimmune diseases are also associated with DM. None of these new associations, which require replication for confirmation, has been previously reported for any form of myositis. Sufficient numbers of myositis samples are not yet available to allow independent consideration of other myositis phenotypes, and these should be addressed in future investigations.
Although this GWAS had a sample size comparable to similar studies of other autoimmune diseases that did identify significant non-MHC signals, no genetic signals with a genome-wide level of significance were observed outside of the MHC. This may be due to a relatively weaker genetic influence and stronger environmental influence on DM susceptibility compared to other autoimmune diseases, or it could be a reflection of disease heterogeneity [1].
By focusing our analysis on a subset of SNPs that are known to be associated with various forms of autoimmunity, we have been able to evaluate these associations in DM without the statistical implications of multiple testing that are associated with a full GWAS analysis. Thus, we have provided evidence for associations between DM and a number of genes previously identified as risk factors for other forms of autoimmunity. These data are consistent with the familial clustering of multiple autoimmune diseases [27], as well as the higher frequencies of certain autoimmune diseases in close relatives of myositis patients [2; 3]. The direction and strength of association with these risk alleles were consistent with published findings in other autoimmune diseases [14; 16; 19; 21; 26]. Nonetheless, we do not believe that our current findings allow us to effectively compare genetic risk scores for DM and other autoimmune diseases at this time.
The strongest non-MHC association of SNPs with DM that are seen in other autoimmune diseases was a suggestive signal on chromosome 2q that was observed in a region containing PLCL1, which is involved in an inositol phospholipid-based intracellular signaling cascade (http://www.omim.org/entry/600597). In this case three typed SNPs (rs7572733, rs1518364, and rs938929) were in strong LD with a PLCL1 SNP (rs6738825), which was previously associated with SLE. PLCL1 is involved not only in the inositol phospholipid-based intracellular signaling cascade, but also regulates the turnover of receptors, and thus it contributes to the maintenance of muscle tone and of gamma-aminobutyric acid–mediated synaptic inhibition [28]. Yet the exact mechanism by which PLCL1 could be associated with the pathogenesis of DM is not clear and will require additional study.
The other autoimmunity genes that are shared with DM encode proteins that current studies suggest are likely to play a role in the pathogenesis of DM. Among the genes found to be common with other autoimmune diseases, BLK encodes a nonreceptor tyrosine kinase of the src family of proto-oncogenes that are typically involved in cell proliferation and differentiation. The BLK protein has a role in B cell receptor signaling and B cell development, and B cells are prominent forms of mononuclear cells found in DM skin and muscle biopsies [29] as well as markers of disease activity [8]. Further evidence for the role of B cells in DM comes from the growing list of disease-specific autoantibodies and from anecdotal reports of the efficacy of anti-B cell therapies [1]. The BLK gene has been associated with SLE [30], systemic sclerosis [31], Sjögren’s syndrome [32], and RA [10], diseases for which B cells are suspected to play important pathogenic roles and with which DM may occasionally form an overlap syndrome. The function of BLK in human B cells and other hematopoietic cells is not well studied, so little information is available regarding the regulation of BLK at the mRNA and protein levels in cell lines. Nonetheless, the rs922483 allele in the BLK gene, which is in LD with rs2736340, is reported to downregulate both BLK mRNA and protein expression in primary human transitional and naïve B cells from cord blood but not from adult B cell subsets, suggesting that involvement of BLK in the risk for autoimmune disease occurs during the early stages of B cell development [33].
CCL21 is one of several chemokine genes clustered on the p-arm of chromosome 9. The protein encoded by this gene inhibits hematopoiesis and stimulates chemotaxis in vitro for thymocytes and activated T cells [34]. The CCL21 protein may also play roles in mediating the homing of lymphocytes to secondary lymphoid organs in angiogenesis [35] and in B cell migration and proliferation [36] in RA. It is a high-affinity functional ligand for chemokine receptor 7 (CCR7) that is expressed on T and B lymphocytes. CCR7 and CCL21 are both expressed on mononuclear cells in the muscles of myositis patients, and CCL21 is also expressed on plasmacytoid dendritic cells, which are important sources for the interferon signature seen in both adult and juvenile DM [37]. CCL21 is also expressed in the extranodal lymphoid microstructures in muscle in juvenile DM [38]. SNPs of CCL21 have been associated with RA, although the functional nature of these SNPs and their possible role in pathogenesis remain to be elucidated [14].
Given the limited information available on the pathogenic mechanisms in DM, as well as the specific functions of the alleles of genes associated with autoimmunity, more investigation is needed to understand the implications of these SNP associations.
The limitations of this study include its moderate statistical power, use of multiple Illumina arrays, and possible heterogeneity from multiple autoantibody phenotypes whose genetic associations sometimes vary from the clinical phenotypes [5], which should all be addressed in future larger confirmatory studies.
Taken together, our findings suggest that DM shares genetic features with other autoimmune diseases, including major genetic contributions in the MHC region and several non-MHC genes that may interact in common functional pathways [39]. This is the first systematic identification of genetic predispositions that are common to autoimmune diseases and that promote the development of DM. An enhanced appreciation of the autoimmune pathogenesis of DM and identification and confirmation of additional genetic risk factors should ultimately lead to molecular profiles that could catalyze novel diagnostic and therapeutic advances.
Supplementary Material
ACKNOWLEDGMENTS
We thank the members of the UK Adult Onset Myositis Immunogenetic Collaboration (AOMIC) for recruiting and enrolling subjects: Drs. Yasmeen Ahmed (Llandudno General Hospital, Wales), Raymond Armstrong (Southampton General Hospital), Robert Bernstein (Manchester Royal Infirmary), Carol Black (Royal Free Hospital, London), Simon Bowman (University Hospital, Birmingham), Ian Bruce (Manchester Royal Infirmary), Robin Butler (Robert Jones & Agnes Hunt Orthopaedic Hospital, Oswestry), John Carty (Lincoln County Hospital), Chandra Chattopadhyay (Wrightington Hospital), Easwaradhas Chelliah (Wrightington Hospital), Fiona Clarke (James Cook University Hospital, Middlesborough), Peter Dawes (Staffordshire Rheumatology Centre, Stoke on Trent), Joseph Devlin (Pinderfields General Hospital, Wakefield), Christopher Edwards (Southampton General Hospital), Paul Emery (Academic Unit of Musculoskeletal Disease, Leeds), John Fordham (South Cleveland Hospital, Middlesborough), Alexander Fraser (Academic Unit of Musculoskeletal Disease, Leeds), Hill Gaston (Addenbrookes Hospital, Cambridge), Patrick Gordon (Kings College Hospital, London), Bridget Griffiths (Freeman Hospital, Newcastle), Harsha Gunawardena (Frenchay Hospital, Bristol), Frances Hall (Addenbrookes Hospital, Cambridge), Beverley Harrison (North Manchester General Hospital), Elaine Hay (Staffordshire Rheumatology Centre, Stoke on Trent), Lesley Horden (Dewsbury District General Hospital), John Isaacs (Freeman Hospital, Newcastle), Adrian Jones (Nottingham University Hospital), Sanjeet Kamath (Staffordshire Rheumatology Centre, Stoke on Trent), Thomas Kennedy (Royal Liverpool Hospital), George Kitas (Dudley Group Hospitals Trust, Birmingham), Peter Klimiuk (Royal Oldham Hospital), Sally Knights (Yeovil District Hospital, Somerset), John Lambert (Doncaster Royal Infirmary), Peter Lanyon (Queens Medical Centre, Nottingham), Ramasharan Laxminarayan (Queens Hospital, Burton Upon Trent), Bryan Lecky (Walton Neuroscience Centre, Liverpool), Raashid Luqmani (Nuffield Orthopaedic Centre, Oxford), Jeffrey Marks (Steeping Hill Hospital, Stockport), Micheal Martin (St James University Hospital, Leeds), Dennis McGonagle (Academic Unit of Musculoskeletal Disease, Leeds), Neil McHugh (Royal National Hospital for Rheumatic Diseases, Bath), Francis McKenna (Trafford General Hospital, Manchester), John McLaren (Cameron Hospital, Fife), Michael McMahon (Dunfries & Galloway Royal Infirmary, Scotland), Euan McRorie (Western General Hospital, Edinburgh), Peter Merry (Norfolk & Norwich University Hospital), Sarah Miles (Dewsbury & District General Hospital), James Miller (Royal Victoria Hospital, Newcastle), Anne Nicholls (West Suffolk Hospital, Bury St Edmunds), Jennifer Nixon (Countess of Chester Hospital), Voon Ong (Royal Free Hospital, London), Katherine Over (Countess of Chester Hospital), John Packham (Staffordshire Rheumatology Centre, Stoke on Trent), Nicolo Pipitone (Kings College Hospital, London), Michael Plant (South Cleveland Hospital, Middlesborough), Gillian Pountain (Hinchingbrooke Hospital, Huntington), Thomas Pullar (Ninewells Hospital, Dundee), Mark Roberts (Salford Royal Foundation Trust), Paul Sanders (Wythenshawe Hospital, Manchester), David Scott (Norfolk & Norwich University Hospital), David Scott (Kings College Hospital, London), Michael Shadforth (Staffordshire Rheumatology Centre, Stoke on Trent), Thomas Sheeran (Cannock Chase Hospital), Arul Srinivasan (Boomfield Hospital, Chelmsford) David Swinson (Wrightington Hospital), Lee-Suan Teh (Royal Blackburn Hospital), Michael Webley (Stoke Manderville Hospital, Aylesbury), Brian Williams (University Hospital of Wales), and Jonathan Winer (Queen Elizabeth Hospital, Birmingham).
We are in debt to all the local research coordinators and principal investigators who contributed to the UK Juvenile Dermatomyositis Cohort Study, including Dr. Liza McCann, Mr. Ian Roberts, and Ms. Louise Hanna (The Royal Liverpool Children’s Hospital, Alder Hey, Liverpool); Dr. Phil Riley, Dr. Eileen Baildam, and Ms. Ann McGovern (Royal Manchester Children’s Hospital, Manchester); Dr. Clive Ryder and Mrs. Janis Scott (Birmingham Children’s Hospital, Birmingham); Dr. Sue Wyatt and Mrs. Gillian Jackson (Leeds General Infirmary, Leeds); Dr. Joyce Davidson, Dr. Janet Gardner-Medwin, and Ms. Sue Ferguson (The Royal Hospital for Sick Children, Yorkhill, Glasgow); Dr. Mark Friswell, Professor Helen Foster, Mrs. Alison Swift, Dr. Sharmila Jandial, and Ms. Vicky Stevenson (Great North Children’s Hospital, Newcastle); Dr. Helen Venning and Mrs. Elizabeth Stretton (Queens Medical Centre, Nottingham); Professor Lucy Wedderburn, Dr. Clarissa Pilkington, Dr. N. Hasson, Mrs. Sue Maillard, Ms. Elizabeth Halkon, Ms. Virginia Brown, Ms. Audrey Juggins, Dr. Sally Smith, Mrs. Sian Lunt, Dr. Elli Enayat, Mrs. Hemlata Varsani, and Miss Laura Beard (Great Ormond Street Hospital, London); and Dr. Kevin Murray (Princess Margaret Hospital, Perth, Western Australia).
We thank members of the United States Childhood Myositis Heterogeneity Study Group who contributed to this study: Drs. David Sherry (Children’s Hospital of Philadelphia, Philadelphia, PA), Carol A. Wallace (Children’s Medical Center, Seattle, WA), Carol B. Lindsley (University of Kansas, Kansas City, KS), Steven W. George (Ellicott City, MD), Judyann C. Olson (Medical College of Wisconsin, Milwaukee, WI), Lawrence S. Zemel (Connecticut Children’s Hospital, Hartford, CT), Catherine A. Bingham (Hershey Medical Center, Hershey, PA), Terri H. Finkel (Children’s Hospital of Philadelphia, Philadelphia, PA), Harry L. Gewanter (Richmond, VA), Lisa Imundo (Columbia University, New York, NY), Chester P. Oddis (University of Pittsburgh, Pittsburgh, PA), Scott A. Vogelgesang (Walter Reed Army Medical Center, Washington, DC), Barbara S. Adams (University of Michigan, Ann Arbor, MI), Gail D. Cawkwell (All Children’s Hospital, St. Petersburg, FL), Donald P. Goldsmith (St. Christopher’s Hospital for Children, Philadelphia, PA), Michael Henrickson (Children’s Hospital, Madera, CA), Ellen A. Goldmuntz (Children’s National Medical Center, Washington, DC), Ildy M. Katona (Uniformed Services University, Bethesda, MD), and Patience H. White (George Washington University, Washington, DC).
We are indebted to Dr. Javier Martin of Granada Spain for supplying Spanish control data and to Dr. Peter Novota at the Institute of Rheumatology, Prague, Czech Republic for supplying Czech controls. We thank Dr. Younghun Han (MD Anderson Cancer Center) for statistical support; Miss Hazel Platt (Centre for Integrated Genomic Medical Research, University of Manchester), Mrs. Fiona Marriage (Centre for Integrated Genomic Medical Research, University of Manchester), and Drs. Maryam Dastmalchi and Eva Jemseby (Karolinska Institutet, Stockholm) for technical support; and Mr. Paul New (Salford Royal Foundation Trust) for ethical and technical support.
We thank Elaine Remmers (National Institute of Arthritis and Musculoskeletal and Skin Diseases, Bethesda, MD) and Douglas Bell (National Institute of Environmental Health Sciences, Research Triangle Park, NC) of the National Institutes of Health for their critical review of the manuscript. We used genome-wide association data generated by the Wellcome Trust Case-Control Consortium 2 (WTCCC2 1958 birth cohort).
Finally, we thank all of the patients and their families who contributed to this study.
This study was supported in part by: the Intramural Program of the NIH, National Institute of Environmental Health Sciences (NIEHS Z01ES101074); European Community’s FP6, AutoCure LSHB CT-2006-018661; The UK Myositis Support Group; Arthritis Research UK (18474); The Cure JM Foundation; the European Science Foundation; the Wellcome Trust; the Henry Smith Charity UK; Action Medical UK; and the Swedish Research Council. The Czech cohort was partially supported by the Ministry of Health, Czech Republic (No. 00023728).
Role of the funding sources. The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All members of the writing group had full access to all the data in the study, and authors had final responsibility for the decision to submit for publication.
Footnotes
Disclaimer. The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the institutions with which they are affiliated.
Financial disclosures: The authors have nothing to disclose.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published.
Study conception and design and funding. Miller, Cooper, Vencovsky, Rider, Lundberg, Padyukov, Amos, and Gregersen designed the study and obtained funding.
Acquisition of data. Miller, Cooper, Vencovsky, Rider, Danko, Wedderburn, Lundberg, Padyukov, Pachman, Reed, Ytterberg, Selva-O’Callaghan, Radstake, Isenberg, and Chinoy collected samples. O’Hanlon, Ollier, Lee, Lamb, Pachman, and Gregersen performed sample processing.
Analysis and interpretation of data. O’Hanlon, Peng, Lee, Lamb, Padyukov, Chen, Amos, and Gregersen did the data analysis, interpretation and data management.
Other Myositis Genetics Consortium Study Investigators: Drs. Christopher Denton (Royal Free Hospital, London, UK), David Hilton-Jones (John Radcliffe Hospital, Oxford, UK), Patrick Kiely (St. Georges Hospital, London, UK), Paul H. Plotz, Mark Gourley (National Institute of Arthritis, Musculoskeletal and Skin Diseases, National Institutes of Health, Bethesda, MD), Paul Scheet (MD Anderson Cancer Center, Houston, TX), and Hemlata Varsani (University College London, London, UK).
References
- 1.Rider LG, Miller FW. Deciphering the clinical presentations, pathogenesis, and treatment of the idiopathic inflammatory myopathies. JAMA. 2011;305:183–190. doi: 10.1001/jama.2010.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ginn LR, Lin JP, Plotz PH, Bale SJ, Wilder RL, Mbauya A, Miller FW. Familial autoimmunity in pedigrees of idiopathic inflammatory myopathy patients suggests common genetic risk factors for many autoimmune diseases. Arthritis Rheum. 1998;41:400–405. doi: 10.1002/1529-0131(199803)41:3<400::AID-ART4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 3.Niewold TB, Wu SC, Smith M, Morgan GA, Pachman LM. Familial aggregation of autoimmune disease in juvenile dermatomyositis. Pediatrics. 2011;127:e1239–e1246. doi: 10.1542/peds.2010-3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deitiker P, Atassi MZ. Non-MHC genes linked to autoimmune disease. Crit Rev Immunol. 2012;32:193–285. doi: 10.1615/critrevimmunol.v32.i3.10. [DOI] [PubMed] [Google Scholar]
- 5.Chinoy H, Lamb JA, Ollier WE, Cooper RG. Recent advances in the immunogenetics of idiopathic inflammatory myopathy. Arthritis Res Ther. 2011;13:216. doi: 10.1186/ar3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller FW. Inflammatory Myopathies: Polymyositis, dermatomyositis, and related conditions. In: Koopman W, Moreland L, editors. Arthritis and Allied Conditions, A Textbook of Rheumatology. Philadelphia: Lippincott, Williams and Wilkins; 2005. pp. 1593–1620. [Google Scholar]
- 7.Zong M, Lundberg IE. Pathogenesis, classification and treatment of inflammatory myopathies. Nat Rev Rheumatol. 2011 doi: 10.1038/nrrheum.2011.39. [DOI] [PubMed] [Google Scholar]
- 8.Feldman BM, Rider LG, Reed AM, Pachman LM. Juvenile dermatomyositis and other idiopathic inflammatory myopathies of childhood. Lancet. 2008;371:2201–2212. doi: 10.1016/S0140-6736(08)60955-1. [DOI] [PubMed] [Google Scholar]
- 9.Bohan A, Peter JB, Bowman RL, Pearson CM. Computer-assisted analysis of 153 patients with polymyositis and dermatomyositis. Medicine (Baltimore ) 1977;56:255–286. doi: 10.1097/00005792-197707000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Gregersen PK, Amos CI, Lee AT, Lu Y, Remmers EF, Kastner DL, Seldin MF, Criswell LA, Plenge RM, Holers VM, Mikuls TR, Sokka T, Moreland LW, Bridges SL, Jr, Xie G, Begovich AB, Siminovitch KA. REL, encoding a member of the NF-kappaB family of transcription factors, is a newly defined risk locus for rheumatoid arthritis. Nat Genet. 2009;41:820–823. doi: 10.1038/ng.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Padyukov L, Seielstad M, Ong RT, Ding B, Ronnelid J, Seddighzadeh M, Alfredsson L, Klareskog L. A genome-wide association study suggests contrasting associations in ACPA-positive versus ACPA-negative rheumatoid arthritis. Ann Rheum Dis. 2011;70:259–265. doi: 10.1136/ard.2009.126821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cornelis MC, Agrawal A, Cole JW, Hansel NN, Barnes KC, Beaty TH, Bennett SN, Bierut LJ, Boerwinkle E, Doheny KF, Feenstra B, Feingold E, Fornage M, Haiman CA, Harris EL, Hayes MG, Heit JA, Hu FB, Kang JH, Laurie CC, Ling H, Manolio TA, Marazita ML, Mathias RA, Mirel DB, Paschall J, Pasquale LR, Pugh EW, Rice JP, Udren J, van Dam RM, Wang X, Wiggs JL, Williams K, Yu K. The Gene, Environment Association Studies consortium (GENEVA): maximizing the knowledge obtained from GWAS by collaboration across studies of multiple conditions. Genet Epidemiol. 2010;34:364–372. doi: 10.1002/gepi.20492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stahl EA, Raychaudhuri S, Remmers EF, Xie G, Eyre S, Thomson BP, Li Y, Kurreeman FA, Zhernakova A, Hinks A, Guiducci C, Chen R, Alfredsson L, Amos CI, Ardlie KG, Barton A, Bowes J, Brouwer E, Burtt NP, Catanese JJ, Coblyn J, Coenen MJ, Costenbader KH, Criswell LA, Crusius JB, Cui J, de Bakker PI, De Jager PL, Ding B, Emery P, Flynn E, Harrison P, Hocking LJ, Huizinga TW, Kastner DL, Ke X, Lee AT, Liu X, Martin P, Morgan AW, Padyukov L, Posthumus MD, Radstake TR, Reid DM, Seielstad M, Seldin MF, Shadick NA, Steer S, Tak PP, Thomson W, van der Helm-van Mil AH, van der Horst-Bruinsma IE, van der Schoot CE, van Riel PL, Weinblatt ME, Wilson AG, Wolbink GJ, Wordsworth BP, Wijmenga C, Karlson EW, Toes RE, de VN, Begovich AB, Worthington J, Siminovitch KA, Gregersen PK, Klareskog L, Plenge RM. Genome-wide association study meta-analysis identifies seven new rheumatoid arthritis risk loci. Nat Genet. 2010;42:508–514. doi: 10.1038/ng.582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terao C, Yamada R, Ohmura K, Takahashi M, Kawaguchi T, Kochi Y, Okada Y, Nakamura Y, Yamamoto K, Melchers I, Lathrop M, Mimori T, Matsuda F. The human AIRE gene at chromosome 21q22 is a genetic determinant for the predisposition to rheumatoid arthritis in Japanese population. Hum Mol Genet. 2011;20:2680–2685. doi: 10.1093/hmg/ddr161. [DOI] [PubMed] [Google Scholar]
- 16.Flesher DL, Sun X, Behrens TW, Graham RR, Criswell LA. Recent advances in the genetics of systemic lupus erythematosus. Expert Rev Clin Immunol. 2010;6:461–479. doi: 10.1586/eci.10.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramos PS, Criswell LA, Moser KL, Comeau ME, Williams AH, Pajewski NM, Chung SA, Graham RR, Zidovetzki R, Kelly JA, Kaufman KM, Jacob CO, Vyse TJ, Tsao BP, Kimberly RP, Gaffney PM, Alarcon-Riquelme ME, Harley JB, Langefeld CD. A comprehensive analysis of shared loci between systemic lupus erythematosus (SLE) and sixteen autoimmune diseases reveals limited genetic overlap. PLoS Genet. 2011;7:e1002406. doi: 10.1371/journal.pgen.1002406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooper JD, Walker NM, Smyth DJ, Downes K, Healy BC, Todd JA. Follow-up of 1715 SNPs from the Wellcome Trust Case Control Consortium genome-wide association study in type I diabetes families. Genes Immun. 2009;10(Suppl 1):S85–S94. doi: 10.1038/gene.2009.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barrett JC, Clayton DG, Concannon P, Akolkar B, Cooper JD, Erlich HA, Julier C, Morahan G, Nerup J, Nierras C, Plagnol V, Pociot F, Schuilenburg H, Smyth DJ, Stevens H, Todd JA, Walker NM, Rich SS. Genome-wide association study and meta-analysis find that over 40 loci affect risk of type 1 diabetes. Nat Genet. 2009;41:703–707. doi: 10.1038/ng.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Swafford AD, Howson JM, Davison LJ, Wallace C, Smyth DJ, Schuilenburg H, Maisuria-Armer M, Mistry T, Lenardo MJ, Todd JA. An allele of IKZF1 (Ikaros) conferring susceptibility to childhood acute lymphoblastic leukemia protects against type 1 diabetes. Diabetes. 2011;60:1041–1044. doi: 10.2337/db10-0446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barrett JC, Hansoul S, Nicolae DL, Cho JH, Duerr RH, Rioux JD, Brant SR, Silverberg MS, Taylor KD, Barmada MM, Bitton A, Dassopoulos T, Datta LW, Green T, Griffiths AM, Kistner EO, Murtha MT, Regueiro MD, Rotter JI, Schumm LP, Steinhart AH, Targan SR, Xavier RJ, Libioulle C, Sandor C, Lathrop M, Belaiche J, Dewit O, Gut I, Heath S, Laukens D, Mni M, Rutgeerts P, Van GA, Zelenika D, Franchimont D, Hugot JP, de VM, Vermeire S, Louis E, Cardon LR, Anderson CA, Drummond H, Nimmo E, Ahmad T, Prescott NJ, Onnie CM, Fisher SA, Marchini J, Ghori J, Bumpstead S, Gwilliam R, Tremelling M, Deloukas P, Mansfield J, Jewell D, Satsangi J, Mathew CG, Parkes M, Georges M, Daly MJ. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn's disease. Nat Genet. 2008;40:955–962. doi: 10.1038/NG.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franke A, McGovern DP, Barrett JC, Wang K, Radford-Smith GL, Ahmad T, Lees CW, Balschun T, Lee J, Roberts R, Anderson CA, Bis JC, Bumpstead S, Ellinghaus D, Festen EM, Georges M, Green T, Haritunians T, Jostins L, Latiano A, Mathew CG, Montgomery GW, Prescott NJ, Raychaudhuri S, Rotter JI, Schumm P, Sharma Y, Simms LA, Taylor KD, Whiteman D, Wijmenga C, Baldassano RN, Barclay M, Bayless TM, Brand S, Buning C, Cohen A, Colombel JF, Cottone M, Stronati L, Denson T, de VM, D'Inca R, Dubinsky M, Edwards C, Florin T, Franchimont D, Gearry R, Glas J, Van GA, Guthery SL, Halfvarson J, Verspaget HW, Hugot JP, Karban A, Laukens D, Lawrance I, Lemann M, Levine A, Libioulle C, Louis E, Mowat C, Newman W, Panes J, Phillips A, Proctor DD, Regueiro M, Russell R, Rutgeerts P, Sanderson J, Sans M, Seibold F, Steinhart AH, Stokkers PC, Torkvist L, Kullak-Ublick G, Wilson D, Walters T, Targan SR, Brant SR, Rioux JD, D'Amato M, Weersma RK, Kugathasan S, Griffiths AM, Mansfield JC, Vermeire S, Duerr RH, Silverberg MS, Satsangi J, Schreiber S, Cho JH, Annese V, Hakonarson H, Daly MJ, Parkes M. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn's disease susceptibility loci. Nat Genet. 2010;42:1118–1125. doi: 10.1038/ng.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amre DK, Mack DR, Morgan K, Israel D, Deslandres C, Seidman EG, Lambrette P, Costea I, Krupoves A, Fegury H, Dong J, Xhu Z, Grimard G, Levy E. Association between genome-wide association studies reported SNPs and pediatric-onset Crohn's disease in Canadian children. Hum Genet. 2010;128:131–135. doi: 10.1007/s00439-010-0835-2. [DOI] [PubMed] [Google Scholar]
- 24.Cooper JD, Simmonds MJ, Walker NM, Burren O, Brand OJ, Guo H, Wallace C, Stevens H, Coleman G, Franklyn JA, Todd JA, Gough SC. Seven newly identified loci for autoimmune thyroid disease. Hum Mol Genet. 2012;21:5202–5208. doi: 10.1093/hmg/dds357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dubois PC, Trynka G, Franke L, Hunt KA, Romanos J, Curtotti A, Zhernakova A, Heap GA, Adany R, Aromaa A, Bardella MT, van den Berg LH, Bockett NA, de la Concha EG, Dema B, Fehrmann RS, Fernandez-Arquero M, Fiatal S, Grandone E, Green PM, Groen HJ, Gwilliam R, Houwen RH, Hunt SE, Kaukinen K, Kelleher D, Korponay-Szabo I, Kurppa K, MacMathuna P, Maki M, Mazzilli MC, McCann OT, Mearin ML, Mein CA, Mirza MM, Mistry V, Mora B, Morley KI, Mulder CJ, Murray JA, Nunez C, Oosterom E, Ophoff RA, Polanco I, Peltonen L, Platteel M, Rybak A, Salomaa V, Schweizer JJ, Sperandeo MP, Tack GJ, Turner G, Veldink JH, Verbeek WH, Weersma RK, Wolters VM, Urcelay E, Cukrowska B, Greco L, Neuhausen SL, McManus R, Barisani D, Deloukas P, Barrett JC, Saavalainen P, Wijmenga C, van Heel DA. Multiple common variants for celiac disease influencing immune gene expression. Nat Genet. 2010;42:295–302. doi: 10.1038/ng.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Jager PL, Jia X, Wang J, de Bakker PI, Ottoboni L, Aggarwal NT, Piccio L, Raychaudhuri S, Tran D, Aubin C, Briskin R, Romano S, Baranzini SE, McCauley JL, Pericak-Vance MA, Haines JL, Gibson RA, Naeglin Y, Uitdehaag B, Matthews PM, Kappos L, Polman C, McArdle WL, Strachan DP, Evans D, Cross AH, Daly MJ, Compston A, Sawcer SJ, Weiner HL, Hauser SL, Hafler DA, Oksenberg JR. Meta-analysis of genome scans and replication identify CD6, IRF8 and TNFRSF1A as new multiple sclerosis susceptibility loci. Nat Genet. 2009;41:776–782. doi: 10.1038/ng.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhernakova A, van Diemen CC, Wijmenga C. Detecting shared pathogenesis from the shared genetics of immune-related diseases. Nat Rev Genet. 2009;10:43–55. doi: 10.1038/nrg2489. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe M, Maemura K, Kanbara K, Tamayama T, Hayasaki H. GABA and GABA receptors in the central nervous system and other organs. Int Rev Cytol. 2002;213:1–47. doi: 10.1016/s0074-7696(02)13011-7. [DOI] [PubMed] [Google Scholar]
- 29.Nagaraju K, Lundberg IE. Polymyositis and dermatomyositis: pathophysiology. Rheum Dis Clin North Am. 2011;37:159–171. doi: 10.1016/j.rdc.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Deng Y, Tsao BP. Genetic susceptibility to systemic lupus erythematosus in the genomic era. Nat Rev Rheumatol. 2010;6:683–692. doi: 10.1038/nrrheum.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coustet B, Dieude P, Guedj M, Bouaziz M, Avouac J, Ruiz B, Hachulla E, Diot E, Cracowski JL, Tiev K, Sibilia J, Mouthon L, Frances C, Amoura Z, Carpentier P, Cosnes A, Meyer O, Kahan A, Boileau C, Chiocchia G, Allanore Y. C8orf13-BLK is a genetic risk locus for systemic sclerosis and has additive effects with BANK1: Results from a large french cohort and meta-analysis. Arthritis Rheum. 2011;63:2091–2096. doi: 10.1002/art.30379. [DOI] [PubMed] [Google Scholar]
- 32.Nordmark G, Kristjansdottir G, Theander E, Appel S, Eriksson P, Vasaitis L, Kvarnstrom M, Delaleu N, Lundmark P, Lundmark A, Sjowall C, Brun JG, Jonsson MV, Harboe E, Goransson LG, Johnsen SJ, Soderkvist P, Eloranta ML, Alm G, Baecklund E, Wahren-Herlenius M, Omdal R, Ronnblom L, Jonsson R, Syvanen AC. Association of EBF1, FAM167A(C8orf13)-BLK and TNFSF4 gene variants with primary Sjogren's syndrome. Genes Immun. 2011;12:100–109. doi: 10.1038/gene.2010.44. [DOI] [PubMed] [Google Scholar]
- 33.Simpfendorfer KR, Olsson LM, Manjarrez ON, Khalili H, Simeone AM, Katz MS, Lee AT, Diamond B, Gregersen PK. The autoimmunity-associated BLK haplotype exhibits cis-regulatory effects on mRNA and protein expression that are prominently observed in B cells early in development. Hum Mol Genet. 2012;21:3918–3925. doi: 10.1093/hmg/dds220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nandagopal S, Wu D, Lin F. Combinatorial guidance by CCR7 ligands for T lymphocytes migration in co-existing chemokine fields. PLoS One. 2011;6:e18183. doi: 10.1371/journal.pone.0018183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pickens SR, Chamberlain ND, Volin MV, Pope RM, Talarico NE, Mandelin AM, Shahrara S. Role of the CCL21 and CCR7 pathways in rheumatoid arthritis angiogenesis. Arthritis Rheum. 2012;64:2471–2481. doi: 10.1002/art.34452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nanki T, Takada K, Komano Y, Morio T, Kanegane H, Nakajima A, Lipsky PE, Miyasaka N. Chemokine receptor expression and functional effects of chemokines on B cells: implication in the pathogenesis of rheumatoid arthritis. Arthritis Res Ther. 2009;11:R149. doi: 10.1186/ar2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khanna S, Reed AM. Immunopathogenesis of juvenile dermatomyositis. Muscle Nerve. 2010;41:581–592. doi: 10.1002/mus.21669. [DOI] [PubMed] [Google Scholar]
- 38.Lopez de Padilla CM, Vallejo AN, Lacomis D, McNallan K, Reed AM. Extranodal lymphoid microstructures in inflamed muscle and disease severity of new-onset juvenile dermatomyositis. Arthritis Rheum. 2009;60:1160–1172. doi: 10.1002/art.24411. [DOI] [PubMed] [Google Scholar]
- 39.Eleftherohorinou H, Wright V, Hoggart C, Hartikainen AL, Jarvelin MR, Balding D, Coin L, Levin M. Pathway analysis of GWAS provides new insights into genetic susceptibility to 3 inflammatory diseases. PLoS One. 2009;4:e8068. doi: 10.1371/journal.pone.0008068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramos PS, Criswell LA, Moser KL, Comeau ME, Williams AH, Pajewski NM, Chung SA, Graham RR, Zidovetzki R, Kelly JA, Kaufman KM, Jacob CO, Vyse TJ, Tsao BP, Kimberly RP, Gaffney PM, Alarcon-Riquelme ME, Harley JB, Langefeld CD. A comprehensive analysis of shared loci between systemic lupus erythematosus (SLE) and sixteen autoimmune diseases reveals limited genetic overlap. PLoS Genet. 2011;7:e1002406. doi: 10.1371/journal.pgen.1002406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abelson AK, Delgado-Vega AM, Kozyrev SV, Sanchez E, Velazquez-Cruz R, Eriksson N, Wojcik J, Linga Reddy MV, Lima G, D'Alfonso S, Migliaresi S, Baca V, Orozco L, Witte T, Ortego-Centeno N, Abderrahim H, Pons-Estel BA, Gutierrez C, Suarez A, Gonzalez-Escribano MF, Martin J, Alarcon-Riquelme ME. STAT4 associates with systemic lupus erythematosus through two independent effects that correlate with gene expression and act additively with IRF5 to increase risk. Ann Rheum Dis. 2009;68:1746–1753. doi: 10.1136/ard.2008.097642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eyre S, Bowes J, Diogo D, Lee A, Barton A, Martin P, Zhernakova A, Stahl E, Viatte S, McAllister K, Amos CI, Padyukov L, Toes RE, Huizinga TW, Wijmenga C, Trynka G, Franke L, Westra HJ, Alfredsson L, Hu X, Sandor C, de Bakker PI, Davila S, Khor CC, Heng KK, Andrews R, Edkins S, Hunt SE, Langford C, Symmons D, Concannon P, Onengut-Gumuscu S, Rich SS, Deloukas P, Gonzalez-Gay MA, Rodriguez-Rodriguez L, Arlsetig L, Martin J, Rantapaa-Dahlqvist S, Plenge RM, Raychaudhuri S, Klareskog L, Gregersen PK, Worthington J. High-density genetic mapping identifies new susceptibility loci for rheumatoid arthritis. Nat Genet. 2012;44:1336–1340. doi: 10.1038/ng.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.