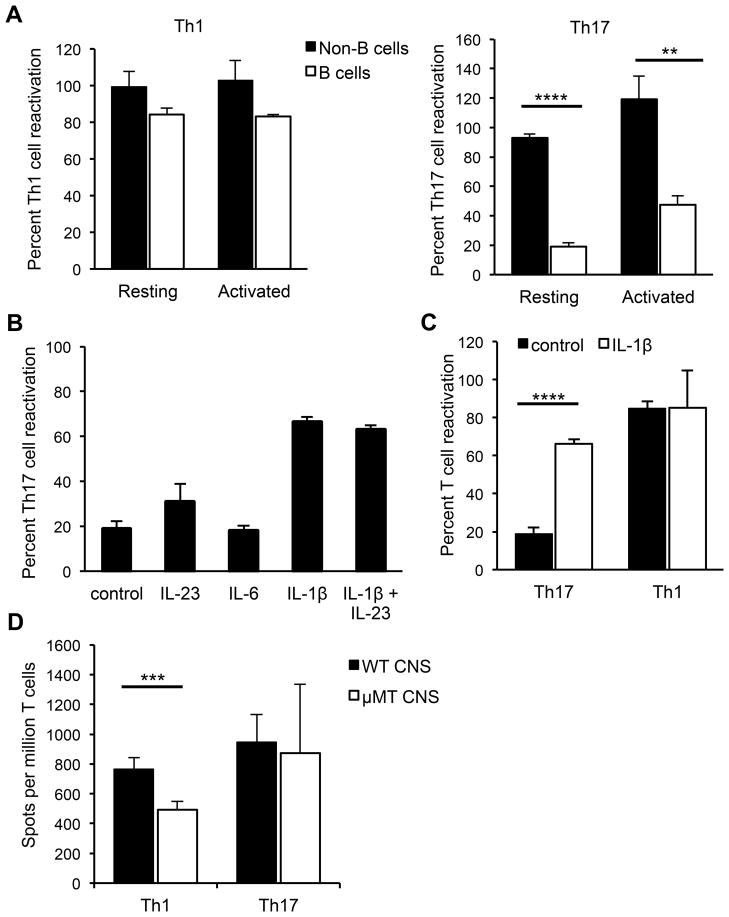
Figure 5. Resting B cells reactivate Th1 but not Th17 cells in vitro.
(A) Splenocytes from naïve mice were FACS-sorted into B cell (CD19+CD43−) or non-B cell (CD19−CD43+) fractions and used as APCs in ELISPOT assays with sorted CD4+ MOG-specific Th1 or Th17 cells to detect IL-17- or IFN-γ-producing cells responding to MOG97-114. Th1 or Th17 cells were co-cultured overnight with B or non-B cells, with or without rMOG. The APCs were used either directly (resting) or were stimulated with LPS and anti-CD40 (activated) prior to culture with T cells. (B) Th17 cells were co-cultured with splenic B cells and rMOG either alone, with IL-23, IL-6, IL-1β or IL-23 and IL-1β. (C) Th17 or Th1 cells were co-cultured with resting spleen B cells with or without IL-1β. Control indicates the absence of added cytokine. (A–C) In each experiment, data are normalized to the number of antigen-specific spots obtained by co-culture of either Th1 or Th17 cells with bulk naïve splenocytes (100%). Data (means ± SEM) are pooled from at least 3 independent experiments. (D) Sorted MOG-specific Th1 or Th17 cells were co-cultured with or without rMOG, with bulk CNS cells from naïve WT or μMT mice that were normalized to ensure equal numbers of non-B APCs in all wells, as described in Materials and Methods. Numbers of antigen-specific spots per million T cells are shown. Data (means ± SEM) are pooled from at least 4 independent experiments. **p < 0.01; ***p < 0.001; ****p < 0.0001, Student’s t test.