Fig. 1.
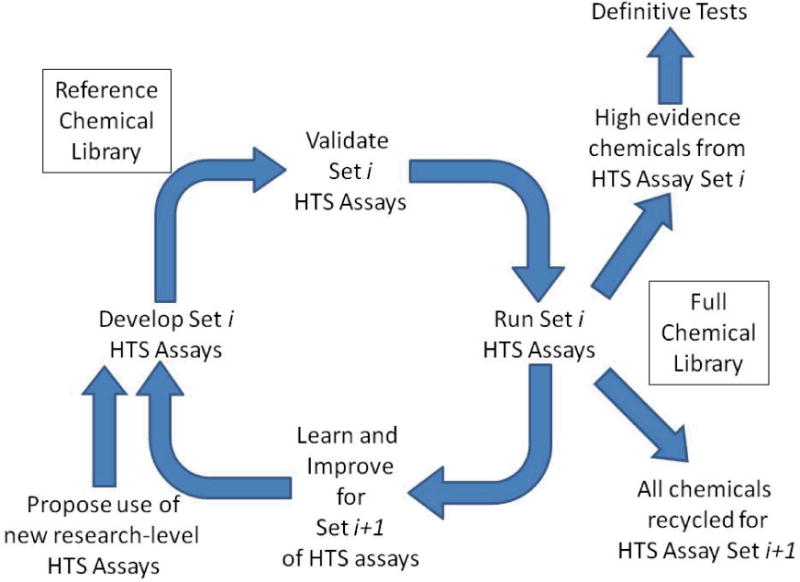
Conceptual model of a continuously improving battery of HTS assays to be used for prioritization. A library of chemicals of interest is identified. For each MOA of concern, in vitro assays that have been identified in a research setting are moved into an HTS platform suitable for screening large chemical libraries. These assays are then validated based on screening a set of reference chemicals. Validation depends on showing that the new assays are reliable (reproducible, giving good signal to background, and low well-to-well variation, etc.; and relevant, i.e. the results on the reference chemicals are in accord with what is known about their activity in the molecular pathway being probed with the assay, and with results in the in vivo definitive test). After validation, the full chemical library is tested. Based on what is learned from testing the library, and from related scientific studies, additional HTS assays for the MOA, or improved versions of the current assays will be introduced and those new assays, in turn, will be validated using the reference chemicals. Subsequently, the full chemical library will be rescreened. “High Evidence” chemicals, i.e. those whose activity in the KE of the MOA being probed is strongly supported by the assay data, will be recommended to be run in more definitive tests. An approach similar to this has been proposed for the U.S. EPA’s EDSP21 approach (U.S. EPA 2011a).
