Abstract
Background
A fundamental obstacle to using retroviral-mediated gene transfer (GT) to treat human diseases is the relatively low transduction levels that have been achieved in clinically relevant human cells. We previously showed that performing GT in utero overcomes this obstacle and results in significant levels of transduction within multiple fetal organs, with different tissues exhibiting optimal transduction at different developmental stages. We undertook the present study aiming to elucidate the mechanism for this age-dependent transduction, testing the two factors that we hypothesized could be responsible: (i) the proliferative status of the tissue at the time of GT and (ii) the expression level of the amphotropic PiT-2 receptor.
Methods
Immunofluorescence was performed on tissues from sheep of varying developmental stages to assess the proliferative status of the predominant cells within each organ as a function of age. After developing an enzyme-linked immunosorbent assay (ELISA) and a quantitative reverse transcription chain reaction (qRT-PCR) assay, we then quantified PiT-2 expression at the protein and mRNA levels, respectively.
Results
The results obtained indicate that the proliferative status of organs at the time of fetal GT is not the major determinant governing transduction efficiency. By contrast, our ELISA and qRT-PCR analyses demonstrated that PiT-2 mRNA and protein levels vary with gestational age, correlating with the observed differences in transduction efficiency.
Conclusions
The findings of the present study explain the age-related differences that we previously observed in transduction efficiency after in utero GT. They also suggest it may be possible to achieve relatively selective GT to specific tissues by performing in utero GT when levels of PiT-2 are maximal in the desired target organ.
Keywords: fetal gene therapy, retroviral vector, transduction, developmental regulation, PiT-2 receptor
Introduction
As of mid-2009, over 20% of all clinical gene therapy trials employed retroviral vectors as agents for therapeutic gene transfer. Among the retroviruses, amphotropic pseudotyped vectors derived from the murine γ-retroviruses (A-MRV) have been used extensively as gene transfer agents for both preclinical and clinical gene therapy trials [1,2]. Binding of the retrovirus to the target cell surface and the cycling status of the target cell are two factors known to be critical for efficient and stable transduction with γ-retroviral vectors [3]. Studies employing a variety of transduction protocols in adult humans [4–6] and primate [7,8] studies have suggested that a fundamental obstacle to the successful application of gene therapy to many genetic diseases is the low efficiency with which amphotropic γ-retroviral vectors transduce clinically relevant cell types, such as hematopoietic stem cells (HSC).
The 71-kDa transmembrane protein, PiT-2, primarily functions as a sodium-dependent phosphate (Na+/Pi) transporter to maintain cellular homeostasis [9–11]. However, several studies aiming to increase the transduction efficiency of A-MRV have suggested that the level of PiT-2 expression correlates with the ability of A-MRV to attach to (and be internalized into) the target cells in various animal models, such as mice, sheep and primates [4–8]. As a result of its indispensible role in transduction with A-MRV, PiT-2 has been designated as the amphotropic retroviral receptor, or Ampho-R. The ability of PiT-2 protein to function as a specific retrovirus receptor has been attributed to amino acid residues that differ between the receptors of the various species [12]. The gene encoding the PiT-2 protein, Slc20a2 (solute carrier family 20) is broadly expressed in several tissues of mammalian species as a housekeeping gene) [13], thereby rendering almost all known mammalian cells susceptible to A-MRV infection [14,15]. At the mRNA level, however, it has been demonstrated that PiT-2 is ubiquitously but differentially expressed within different organs of the body [10,12,16]. This fluctuating expression of PiT-2 results in pronounced differences in the transduction efficiency of various tissues with A-MRV vectors.
In previous studies, we have demonstrated that the efficiency of gene transfer to the major cell types within the various organs of the fetal sheep appears to be dependent on gestational age [17]. Our analyses showed that significantly higher levels of transduction/transgene expression occurred in the liver at earlier gestational ages, whereas, within the lung, transduction efficiency was greatly enhanced at later stages of fetal development. From these findings, we hypothesized that the gestational age-related alterations in the efficiency of retroviral vector transduction within these organs was likely governed by one or both of two mechanisms: (i) the proliferative status of the tissue at the time of gene transfer because the A-MRV-based vectors that we employed in our studies require mitosis for nuclear entry and (ii) the levels of expression of the PiT-2 receptor used by the vector to attach to and enter the target cells. The present studies were thus undertaken aiming to elucidate the major factor determining the age-related alteration in the efficiency of fetal gene transfer.
The results obtained indicate that, although the overall high level of proliferation within each of the developing organs likely explains the relative ease with which cells within these organs can be transduced within the fetus, it cannot explain the alterations in transduction efficiency that we observed as a function of recipient age. By contrast, when the levels of PiT-2 expression within the fetal liver and lung were assessed throughout gestation at both the mRNA [using an ovine-specific quantitative reverse transcription chain reaction (qRT-PCR) assay] and protein [enzyme-linked immunosorbent assay (ELISA)] levels, we found that the levels of PiT-2 mRNA and protein correlated with the alterations that we observed in transduction efficiency within these tissues after the direct intraperitoneal injection of A-MRV vectors into fetal sheep recipients. The present studies thus provide a possible explanation for the age-related differences that we previously observed in the transduction efficiency of cells within the liver and lung after in utero gene transfer (IUGT). They also suggest that it may be possible to achieve relatively selective gene transfer to specific tissues by performing in utero gene therapy when levels of PiT-2 are maximal within the desired target organ.
Materials and methods
Collection of control fetal sheep tissues
The present study was approved by the University of Nevada, Reno Institutional Animal Care and Use Committee. Control fetal sheep were euthanized with an injection of phenytoin at various gestational ages in the range 40–114 days. Fetal liver and lung were then collected in ice-cold Dulbecco’s phosphate-buffered saline (D-PBS) for studies on the proliferative status of organs and the expression of the PiT-2 receptor, as described below. Three normal control fetuses were examined at each gestational stage. For studies involving the assessment of transduction efficiency, fetal sheep were injected intraperitoneally at the indicated ages with helper-free supernatant containing the replication-defective G1Na murine retroviral vector (titer: 1 × 107) as described previously [1,17], in which the NeoR gene encoding neomycin phosphotransferase (NPT) is driven by the constitutively active viral long terminal repeat. Fetuses were then euthanized at 30 days post-vector injection and tissues were collected for analysis as described below.
Preparation of frozen tissue sections
Fetal liver and lung samples were fixed in 4% paraformaldehyde in D-PBS for 2 h at 4 °C. The samples were washed in D-PBS three times for 5 min each and then cryoprotected in 20% sucrose in D-PBS overnight at 4 °C. Before freezing, the tissues were incubated for 1 h in freezing medium consisting of 1 part Tissue-Tek OCT Compound (Sakura Finetek USA, Inc., Torrance, CA, USA) and two parts 20% sucrose in D-PBS. Fetal tissues were then flash frozen in Peel-Away base molds (Thermo-Electron, Waltham, MA, USA) by immersion in 2-methylbutane (Fisher Scientific, Waltham, MA, USA) containing dry ice. A Leica minotome (Leica Microsystems GmbH, Wetzlar, Germany) was used to section each tissue at −20 °C, and 7–10-μm thick cryosections were adhered to Superfrost slides (Fisher Scientific). The slides were allowed to dry at room temperature for at least 1 h and were then stored at −80 °C until use.
Immunofluorescence analysis of proliferative status
Mounted tissue sections were immersed in PBS, and then blocked in PBS containing 10% normal goat serum (NGS); blocked sections were incubated in primary antibody diluted in PBS with 2% NGS overnight at 4 °C. Primary antibodies were: rabbit anti-Ki67 (Lab Vision, Fremont, CA, USA) to label dividing cells; mouse anti-α-fetoprotein (AFP) (BioGenex, San Ramon, CA, USA) to label fetal hepatocytes; mouse anti-cytokeratin (CK) (BioGenex) to label epithelial cells; rabbit anti-NPTII (Upstate, Charlottesville, VA, USA) to label transduced cells; mouse anti-OV-6 (R&D Systems, Minneapolis, MN, USA) to label hepatic progenitors (oval cells); and mouse anti-CD45 (BioGenex) to label hematopoietic cells.
After incubation with the respective primary antibody, slides were washed in PBS with 2% NGS and then incubated with Alexa-conjugated (488, 594 or 633) secondary antibody (Invitrogen, Carlsbad, CA, USA) in PBS with 2% NGS for 1 h at 4 °C. After washing, the nuclei in the sections were then counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (Biogenex). An Olympus Fluoview 1000 confocal microscope system (Olympus America, Melville, NY, USA) was used to visually analyse tissue sections and capture images. Images of ten to 12 sections from each of the tissue sections from each animal were captured and the expression of the proliferative and tissue-specific markers was assessed. The percentage of dividing liver and lung-specific cells was then calculated by dividing the number of AFP+/Ki67+ cells (liver), OV-6+/Ki67+ cells (liver), and CK+/Ki67+ cells (lung) by the total number of AFP+, OV-6+ or CK + cells present within each tissue section. Multiple sections from each liver and the lung of three normal control fetuses at each gestational age were examined. To assess the transduction of oval cells as a function of the age at which IUGT was performed, three sections of the liver from each animal were subjected to dual-staining with antibodies to OV-6 and NPTII and examined on a confocal microscope. Five hundred to 1000 OV-6+ oval cells (red) were identified in each section, and the expression of the transgene product, NPTII (green) was assessed visually. The percentage of oval cells exhibiting transgene expression was then calculated by dividing the number of NPTII + oval cells by the total number of oval cells present within each tissue section.
Digital image acquisition
All images were captured with an Olympus Fluoview 1000 confocal microscopy system using the included Olympus software. Images were then subjected to cropping and minimal global processing, such as brightness and contrast adjustment and colour balance, in Adobe Photoshop CS (Adobe Systems Inc., San Jose, CA, USA).
Culturing cells for protein and rna extraction
PiT-2 expressing Mus dunni tail fibroblasts (MDTF) cell line was cultured in complete Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/ml of penicillin and 100 μg/ml of streptomycin, 600 μg/ml of G418, and 2 mM L-glutamine). These cells were used as a positive control for experiments comparing mRNA and the protein expression levels of PiT-2 within fetal sheep liver and lung, whereas NIH 3 T3 cells (ATCC, Manassas, VA, USA) and OA1 sheep brain-derived fibroblast-like cells (ATCC) were used as negative controls.
Membrane protein extraction from fetal tissues
Liver and lung tissue harvested from the control fetal sheep was dissected to obtain 50–60 mg of each tissue sample. Each sample was then homogenized in 3 ml of DMEM–10% FBS media with a dounce homogenizer for 20–30 s to obtain single cell suspensions. Homogenized cells were then centrifuged at 300g for 7 min and washed twice with 10 ml of PBS. The pellet was resuspended in 3 ml of Lysis buffer [250 mM sucrose, 15 mM Tris–HCl (pH 7.4), 3 mM dithiothreitol (DTT), 2 mM ethylenediaminetetraacetic acid and protease inhibitor cocktail (catalog number: P8340; Sigma, St Louis, MO, USA)] and complete lysis of the sample was then ensured by freeze–thaw and sonication for 30–60 s. The resultant cell lysates were then centrifuged at 800 g for 10 min at 4 °C to separate cellular proteins (cytoplasmic and plasma enriched) from cell nuclei and debris. The supernatant was transferred to 3-ml capacity polycarbonate Beckman ultracentrifuge tubes (Beckman Coulter, Fullerton, CA, USA) and ultracentrifuged at 4 °C for 30 min at 100 000 g. The final pellet, which consists predominantly of plasma-membrane enriched proteins, was then resuspended in 0.5% sodium dodecylsulfate (SDS)/PBS solution with a rotor-stator homogenizer. Extracted membrane proteins were quantified using the EZQ kit (Invitrogen) in accordance with the manufacturer’s instructions and kept at −20 °C until use.
SDS-polyacrylamide gel electrophoresis (PAGE) and western blotting of membrane proteins for PiT-2
Membrane proteins were separated according to their size on a broad molecular weight range precast NuPage 4–12% Bis-Tris gel (Invitrogen) to ascertain the presence of PiT-2 in the extracted fraction. Membrane protein samples (1 μg) were mixed with 1 × NuPage LDS sample buffer containing DTT (Invitrogen) to a final volume of 15 μl, heated for 5 min at 95 °C, and cooled for 2 min at room temperature. Samples were loaded and the gel was run for 35 min at 200 V. A SeeBlue Plus2 Pre-stained Protein Standard (Invitrogen) was also run to determine the size of the separated proteins.
After electrophoresis, proteins were transferred to polyvinylidene fluoride membrane using the X-Cell Blot Module (Invitrogen) in accordance with the manufacturer’s instructions and the transfer was verified by staining with Ponceau S stain (Boston BioProducts, Worcester, MA, USA). Western blotting with a primary antibody to PiT-2 (obtained from Dr Wayne Anderson, NCI, Bethesda, MD, USA) and subsequent horseradish peroxidase (HRP)-conjugated chemiluminescent secondary antibody was then performed. The membrane was rinsed with washing buffer (0.05% Tween-20 in TBS) for 3 min, and blocked with 3% bovine serum albumin (BSA) in washing buffer for 30 min at 4 °C in a shaker. Rabbit anti-PiT-2 antibody was diluted 1:500 in blocking buffer and added in sufficient volume to cover the membrane, and the membrane was then allowed to incubate overnight at 4 °C. The next day, the membrane was washed four times for 5 min each with washing buffer. HRP-conjugated goat anti-rabbit antibody was diluted 1:1000 in blocking buffer and added to the membrane, followed by a 2-h incubation at 4 °C. After four 5-min washes, chemiluminescence super-signal substrate (Supersignal West Dura HRP Detection Kit; Pierce Biotechnology, Inc., Rockford, IL, USA) was continuously flooded over the membrane using a plastic pipette for 3 min. The blot was then carefully wrapped in plastic wrap and photographed with a CCD cooled camera at full-aperture (Olympus America, Melville, NY, USA) in complete darkness without any filters.
ELISA to quantify PiT-2
Protein-level expression of PiT-2 receptor in different fetal tissues throughout gestation was detected by the ELISA technique using a Protein Detector HRP Microwell ELISA Kit (KPL, Gaithersburg, MD, USA) in accordance with the manufacturer’s instructions. Briefly, membrane proteins were diluted to a final concentration of 1 μg/ml with 1 × coating buffer, dispensed as 100 μl into 96-well polysorp microwell plates (NUNC, Rochester, NY, USA), and incubated overnight at 4 °C. Wells were blocked for 15 min at room temperature with 300 μl of 1 × BSA blocking solution. After removal of the blocking solution, the primary antibody, rabbit anti-PiT-2 immunoglobulin (Ig)G (produced by Dr Wayne Anderson, NCI), was diluted 1:500 with diluent solution, 100 μl was added to each well, and the plates were then incubated for 1 h at room temperature. The unbound primary antibody in the wells was removed with three washes of 200 μl of 1 × wash solution. HRP-conjugated anti-rabbit IgG secondary antibody was diluted 1:1000 with HRP Stabilizer, 100 μl was added to each well, and the plates were incubated for 1 h at room temperature. Wells were again washed three times with 200 μl of 1 × wash solution to remove unbound secondary antibody. Next, 100 μl of SureBlue TMB Microwell Substrate (KPL, Inc., Gaithersburg, MD, USA) was dispensed into each well, incubated for 5–10 min, and the plates were read at 690 nm in a microplate ELISA reader (Bio-Rad, Hercules, CA, USA). Protein extracts from PiT-2 over-expressing MDTF cells were included in all experiments as a positive control.
RNA extraction and first-strand cDNA synthesis
Liver and lung tissue collected from the control fetal sheep was immediately placed in RNAlater (RNA Stabilization Reagent; Qiagen, Valencia, CA, USA) and stored at 4 °C until use. Total RNA was extracted with an RNeasy Plus Mini Kit (Qiagen) in accordance with the manufacturer’s instructions. The RNAlater-stabilized tissue samples were cut on a clean surface, and 20–30 mg of each tissue was placed in microcentrifuge tube containing Buffer RLT Plus (Qiagen) (600 μl) with β-mercaptoethanol, and homogenized using a Tissue-Tearor (Biospec Products, Bartlesville, OK, USA) rotor-stator homogenizer. Lysates were centrifuged for 3 min at maximum speed at room temperature. The supernatant was carefully transferred by pipetting and genomic DNA was removed using a gDNA-Eliminator spin column (Qiagen). Flow-through was then combined with 600 μl of 70% ethanol, transferred to an RNeasy silica-gel membrane spin column, and washed with buffer RW1 and RPE. Total RNA was then eluted using 45 μl of RNase-free H2O. Quantity and integrity analysis of the extracted RNA was performed using the Agilent 2100 Bioanalyser (Agilent Technologies, Santa Clara, CA, USA). cDNA was synthesized from total RNA (2 μg) using the Omniscript Reverse Transcription (RT) Kit (Qiagen) in accordance with the manufacturer’s instructions, using random hexamers to prime reverse transcription. cDNA samples were stored at −20 °C until use.
qRT-PCR
The homology research by HomoloGene Search Engine of NCBI suggested that the gene Slc20a2 (PiT-2), is highly conserved among eukaryotes. Ovine glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal reference gene. Therefore, primers and probes were designed accordingly using Primer Express, version 2.0 (Applied Biosystems, Foster City, CA, USA).
A real-time PCR Custom TaqMan Gene Expression Assay (Applied Biosystems, Foster City, CA) was designed using File Builder, version 3.1 (Applied Biosystems) to measure the mRNA expression of ovine Slc20a2 (ovSlc20a2) and ovine GAPDH (ovGAPDH). The sequence of a 600-bp region of the ovSlc20a2 mRNA was previously acquired in our laboratory using degenerate primers (unpublished data1), whereas the mRNA sequence of ovine GAPDH was retrieved from GenBank (GI 2623287). Primers were designed and verified to amplify 72 bp of ovSlc20a2 and a splice site that spans an intron was selected as the target sequence.
The custom TaqMan Gene Expression Assay for ovSlc20a2 consisted of the oligonucleotides: TaqMan MGB-Probe FAM-labelled (5′-AAGATTGTTGCTTCTTGGTTT-3′); forward primer (5′-ACCCAAGGTGTGCAGTGGAT-3′); and reverse primer (5′-ACAGCACGCCAGACATGAAA-3′).
To amplify/detect a 100-bp region of ovGAPDH mRNA, the oligonucleotides employed were: TaqMan MGB-Probe FAM-labelled (5′-AGAAGACTGTGGATGGC-3′); forward primer (5′-AGGGACTTATGACCACTGT-3′); and reverse primer (5′-CCAGTAGAAGCAGGGATGATGT-3′).
All reactions were performed in a final volume of 50 μl using TaqMan Universal PCR Master Mix in accordance with the manufacturer’s instructions in 96-well PCR plates and were sealed with Optical Adhesive Cover (Applied Biosystems). DNA samples were run in triplicate, and real-time PCR amplification data was acquired with an ABI PRISM 7000 Sequence Detection System, version 1.2.3. (Applied Biosystems). The thermal cycler protocol was: stage 1: one repetition of 50.0 °C for 2 min; stage 2: one repetition of 95.0 °C for 10 min; and stage 3: 40 repetitions of 95.0 °C for 15 s, 60 °C for 1 min.
To assess the amplification efficiency of the Custom TaqMan Gene Expression Assays, standard curves were generated for qRT-PCR of ovSLc20a2 and ovGAPDH by employing four-fold serial dilutions, so that the amount of cDNA per reaction was in the range 0.4–100 ng. All reactions were performed in triplicate, and the efficiency of PCR amplification was determined by the equation:
where S is the slope of the best fit line. Relative quantification of PiT-2 mRNA expression levels within the various tissues was performed using a standard curve assay.
Statistical analysis
Statistics of the detected values included calculating the SEM by dividing the mean SD by the square root of sample size (n), which is number of tissues from different gestational ages.
Results
Proliferative status of fetal liver during gestation
In our previous studies, we observed differences in the transduction efficiency among various tissues as a function of the recipient age at the time of IUGT in the fetal sheep model [17]. The viral vector used in for IUGT in the present study was an A-MRV and, as such, requires target cells to be actively proliferating to gain access to genomic DNA as a result of breakdown of the nuclear membrane during mitosis. Based on this requirement, we hypothesized that one possible explanation for the differences that we observed in the optimal gestational age for transduction within the liver and the lung could be that maximal proliferation/expansion within these two tissues occurs at different points during fetal life. Indeed, the differing demands placed upon these two organs during various stages of development support this hypothesis. The liver serves important functions starting early in fetal life and, as such, is rapidly proliferating fairly early in gestation and should therefore be most susceptible to transduction with A-MRV during this period. By contrast, the lungs commence significant expansion only later in gestation, in anticipation of having to fulfil the respiratory needs of the infant upon parturition. It thus stands to reason that the lungs should be more susceptible to retroviral-mediated gene transfer late in gestation, just as we had observed in our previous studies.
To test this hypothesis, we collected tissues from normal healthy sheep fetuses of varying gestational ages and prepared cryosections. We then performed immunofluorescence analyses on these sections simultaneously employing primary antibodies to various tissue cell-specific markers and an antibody to the Ki67 protein that is present during all active phases of the cell cycle but absent in resting cells. The combined use of Ki67 in conjunction with cell-specific markers enabled us to examine whether the proliferative status of the major cell types within these organs during gestation was the primary factor governing the efficiency of retroviral transduction.
In our first experiments, fetal liver sections from sheep (n = 2–3 per age) of gestational ages 52, 65, 80, 85 and 102 days were dual-stained for α-AFP, which is expressed solely in fetal hepatocytes/hepatoblasts, and Ki67 to assess the proliferative status of fetal hepatocytes/hepatoblasts in the liver as a function of gestational age (representative sections shown in Figure 1). Given the inverse correlation between transduction efficiency and gestational age in the fetal liver that we had observed in our previous studies, we anticipated a higher percentage of the AFP + cells would be cycling at earlier stages in gestation. However, our immunofluorescence analyses demonstrated that this was not the case. Rather, we found that the percentage of hepatocytes/hepatoblasts that are actively cycling within the fetal liver was relatively constant at 25 ± 2% on days 52, 65 and 80, but then increased to 53 ± 2% on days 85 and 102 of gestation (Figure 2). Thus, these immunofluorescence analyses of the fetal sheep liver suggest that alterations in the proliferative status of the hepatocytes/hepatoblasts as a function of gestational age cannot explain the alteration in retroviral gene transfer efficiency that we observed in our previous studies.
Figure 1.
Proliferating fetal hepatocytes/hepatoblasts within the liver. Liver sections from fetal sheep of varying gestational ages were dual-stained in red for α-AFP and green for Ki67 to assess the percentage of fetal hepatocytes/hepatoblasts proliferating at each developmental stage. The nuclei in the sections are counterstained blue with DAPI. (A) Representative liver sections at each of the ages examined. Scale bar = 50 μm (× 60 magnification). (B) A high magnification view of a representative section of liver from one of the sheep that received the A-MRV injection at 85 days of gestation is shown, confirming nuclear localization of Ki67 staining and the presence of Ki67 immunoreactivity (green nucleus) in AFP + (red) cells.
Figure 2.
Percentage of dividing cells in the fetal liver increases with gestational age. The percentage of AFP+/Ki67+ proliferating fetal hepatocytes/hepatoblasts within liver sections at each age was quantified by counting 1000 AFP + cells in each section and scoring the percentage that were also positive for Ki67. The results of these analyses are shown graphically. Results are presented as the mean ± SD.
Efficient transduction of multiple cell types within the fetal liver
As can be seen in Figure 1, in addition to fetal hepatocytes, there were also large numbers of small rounded proliferating cells within the liver that appeared to be of hematopoietic origin. This is not unexpected given that one of the primary functions of the early fetal liver is to maintain haematopoiesis until the bone marrow takes over this task late in gestation near birth. In addition, small nonparenchymal cells with morphology suggestive of liver progenitors (oval cells) were also present and labelled with Ki67, indicating that they may also be susceptible to transduction after in utero delivery of A-MRV vectors. Based on these observations, we set out to determine which cell types within the fetal liver, other than hepatocytes, were transduced after IUGT. To accomplish this objective, we prepared cryosections from the liver of sheep that had received IUGT at gestational ages of 55, 61, 73, 76 and 114 days. These sections were then dual-stained for the NPT-II reporter contained within our A-MRV vector, and for OV-6, which is a marker of liver progenitors (oval cells). In addition, tissues of the same animals were also dual-stained for NPT-II and CD45 to identify all hematopoietic cells. These analyses yielded several important results. First, they revealed that the numbers of OV-6+ progenitors present within the fetal liver tended to increase with advancing gestational age. Therefore, despite the fact that the percentage of OV-6+ cells that were proliferatively active as assessed by Ki67 staining remained fairly constant throughout gestation (data not shown), the absolute number of OV-6+ cells that were proliferating increased as a function of gestational age. Not unexpectedly, this led to increased numbers of these progenitor cells transduced later in gestation (Figure 3A). A representative section from an animal that received A-MRV at 55 days of gestation, stained with anti-NPTII (green) and anti-Ov-6 (red), is shown in Figure 3B. With respect to the hematopoietic lineages, the percentages of transgene-expressing cells appeared to fluctuate among the different gestational ages, with no clear pattern emerging that would allow us to make a correlation between gestational age and the rate of transduction of hematopoietic cells present within the fetal liver (data not shown). Consequently, our results suggest that we successfully transduced liver progenitors and hematopoietic lineages via retroviral gene transfer at different times of gestation by direct intraperotoneal injection. Additionally, the presence and transduction efficiency of liver progenitors appears to correlate very well with increasing gestational age.
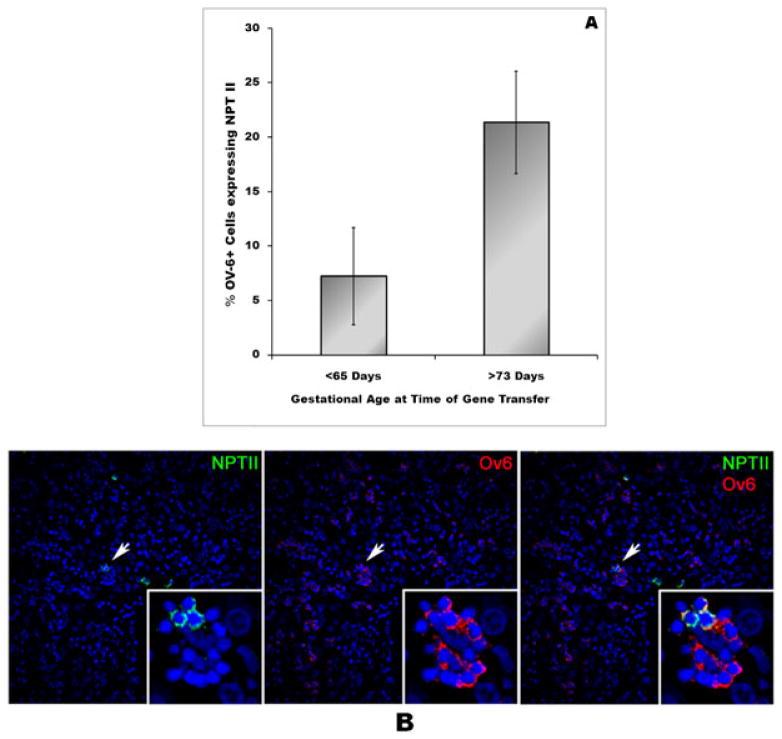
Figure 3.
Transduction efficiency of OV-6+ Liver progenitors (oval cells) after IUGT increases with advancing gestational age. Fetal sheep received an injection of A-MRV at varying gestational ages (55–114 days). Livers were collected and were analyzed by confocal microscopy after dual-staining for the vector-encoded neomycin phosphotransferase II (NPT-II) reporter and for OV-6, a marker of liver progenitors (oval cells) to quantify the percentage of these stem/progenitors transduced after IUGT at each developmental stage. (A) Bar graph depicting the results of counting liver sections from these sheep after dual-staining (for details, see Materials and methods). Bars represent the the mean ± SEM (n = 6 for each age bracket). (B) Representative liver section from a fetal sheep that received an injection of A-MRV at 55 days of gestation, dual-stained for the vector-encoded NPT-II reporter (in green) and for OV-6 (in red). Colocalization of these two signals demonstrates the successful transduction of OV-6+ oval cells after direct intraperitoneal injection of A-MRV during fetal development (×40 magnification).
Proliferative status of fetal lung during gestation
We next extended our analyses to the fetal lung with the purpose of finding a plausible explanation for our observation that gene transfer efficiency in this particular tissue increased with gestational age. Accordingly, frozen fetal lung tissues from sheep at gestational ages of 52, 65, 80, 85 and 102 days were sectioned as 8 μm and stained for Ki67 and CK, which stains epithelial cells in the fetal lung (Figure 4), to determine their proliferative status. We observed that both cytokeratin-positive epithelial cells, as well as several other morphologically distinct cell types within the lung, were dividing, especially within the regions comprising the forming bronchioles and alveoli. Unfortunately, although the number of transgene-expressing cells in the lung after direct intraperitoneal injection of retroviral vector increased in direct correlation with the gestational age, quantification of the percentage of CK+ cells at each age that co-labelled with Ki67 revealed that the proliferative status of these cells remained relatively constant throughout gestation. Consequently, similar to what we had observed in the fetal liver, the proliferative status of the epithelial and other cell types in the fetal lung does not appear to provide an explanation for the change in transduction efficiency that was seen with gestational advancement.
Figure 4.
Proliferative status of epithelial cells within the fetal lung remains relatively constant throughout gestation. Fetal lung sections from sheep of varying gestational ages were dual-stained for CK to identify epithelial cells within the lung, and Ki67 to assess their proliferative status. A representative section of lung at each of the ages examined is presented, showing no correlation between age and proliferative status. Scale bar = 50 μm (× 60 magnification).
Expression of PiT-2 at the mRNA level
Given the lack of correlation between transduction efficiency and proliferative status, we hypothesized that the levels of expression of the PiT-2 receptor used by A-MRV for cell attachment/entry may change within the fetal liver and lung during gestation and thus provide a mechanistic explanation for the observed alterations in transduction efficiency. qRT-PCR is a very sensitive, accurate, and reproducible means of measuring the levels of gene expression. We therefore used qRT-PCR as a first means of quantitating the levels of PiT-2 expression within the liver and lung as a function of gestational age. The gene encoding the PiT-2 receptor protein in humans is Slc20a2. The gene is highly conserved among species; however, the ovine Slc20a2 gene has not yet been completely sequenced and deposited in the NCBI GenBank to enable us to design appropriate primers for qRT-PCR. We therefore designed degenerate primers to amplify and sequence overlapping regions of a portion of the coding region of ovSlc20a2 obtained from fetal liver cDNA. The resulting contigs from the sequencing results were then used as the templates to set up qRT-PCR assays. In addition, as a built-in safeguard, the primers that we employed for these qRT-PCR assays were specifically designed to amplify an exon–exon junction, which spans an intron, and would thus ensure that no signal could be obtained if any minimal genomic DNA contamination was present within our RNA preps.
OvSlc20a2 gene expression in fetal sheep tissues
To detect the functionality and efficiency of the custom-designed primers and probe, we set up validation experiments and performed standard curve analysis, which demonstrated greater than 95% PCR efficiency in our real-time detection assays. Furthermore, we also designed primers and probe for ovGAPDH as an internal reference gene to normalize CT values of standard and unknown cDNA samples. The results of validation and standard curve assays for ovGAPDH resulted in a similar PCR efficiency (> 95%). Having thus validated our qRT-PCR assays, we next examined the expression of ovSlc20a2 (PiT-2) at the mRNA level relative to ovGAPDH within the liver and lung of fetal sheep at a range of gestational ages and in adult sheep. As shown in Figure 5, the level of ovSlc20a2 mRNA is the highest at the earliest gestational age tested (day 52), and then decreases throughout the remainder of gestation, dropping at day 79 to levels six-fold lower than those seen at day 52. Interestingly, at day 102, the levels of expression rise to some degree, although they are still three-fold lower than those seen at day 52. This pattern of ovSlc20a2 mRNA expression in the fetal liver thus parallels our results on retroviral transduction efficiency within this tissue during the same developmental period.
Figure 5.
mRNA expression of amphotropic receptor (PiT-2) in fetal liver decreases with advancing gestational age. PiT-2 mRNA expression levels within the liver at various gestational ages were quantified by qRT-PCR using the comparative CT method with the value at day 58 serving as the calibrator. The ΔΔCT value was first obtained by subtracting the mean ovGAPDH CT value from the mean PiT-2 CT value. The ΔΔCT values were calculated by subtracting the ΔCT at each gestational age by the ΔCT at day 58, which was designated as calibrator. The values of PiT-2N were calculated using the equation for relative quantification. The bars represent the SD of the difference, which was calculated using the equation . Samples were run in triplicate. For details, See Materials and methods.
Similarly, within the fetal lung, the mRNA expression level of ovSlc20a2 increased with increasing recipient age, which is in direct correlation with the retroviral gene transfer efficiency that we observed after IUGT (Figure 6). The lowest amplification occurred at the earliest gestational age (day 52) and ovSlc20a2 gene expression steadily rose as the gestational age increased. Furthermore, expression of ovSlc20a2 mRNA within adult sheep lung was 3.5-fold higher than that observed at earlier times during the prenatal period, suggesting that perhaps the lung may best be targeted either late in gestation, or even after birth, if high levels of transduction and transgene expression are likely to be required to correct the disorder in question.
Figure 6.
mRNA expression of amphotropic receptor (PiT-2) in fetal lung increases with advancing gestational age. PiT-2 mRNA expression levels within the lung at various gestational ages were quantified by qRT-PCR using the comparative CT method with day 52 serving as the calibrator. The ΔΔCT value was first obtained by subtracting the mean ovGAPDH CT value from the mean PiT-2 CT value. The ΔΔCT values were calculated by subtracting the ΔCT at each gestational age by the ΔCT at day 52, which was designated as calibrator. The values of PiT-2N were calculated using the equation for relative quantification. The bars represent the SD of the difference, which was calculated using the equation . Samples were run in triplicate. For further details, see Materials and methods.
Expression of PiT-2 at the protein level
Although the results of our qRT-PCR analyses provided compelling evidence that the levels of expression of PiT-2 could explain, at least in part, our previous observations on transduction efficiency within the lung and liver after IUGT, we next performed studies to confirm that the alterations in the levels of PiT-2 expression seen at the mRNA level were also present at the protein level. Membrane proteins are more challenging than cytoplasmic proteins to extract by conventional methods, and PiT-2 is found in the detergent-insoluble fraction, which makes it far more difficult to isolate from tissues. For this reason, we attempted several different methods to obtain adequate amounts of PiT-2 protein from fetal liver and lung aiming to quantify the levels of expression of this protein during gestation in these tissues. We finally optimized the extraction of PiT-2 using homogenization, freeze–thaw, sonication and ultracentrifugation steps.
No antibodies specific for PiT-2 receptor antigen were commercially available at the time of commencing our research. We had previously generated a rabbit polyclonal antibody specific for the human PiT-2 protein [18–20]. However, it was not known whether this antibody would recognize the ovine homolog of PiT-2. To address this critical question, we performed SDS-PAGE and western blotting analyses. This assay revealed that the anti-human PiT-2 polyclonal antibody was able to efficiently detect the ovine homolog of PiT-2 (Figure 7), confirming that we could use this reagent to quantify the expression levels of PiT-2 in fetal sheep liver and lung.
Figure 7.
Western blotting of fetal sheep tissues to confirm anti-human PiT-2 polyclonal antibody recognizes the ovine homolog. To verify that the anti-human PiT-2 polyclonal antibody recognized the ovine homolog, membrane proteins were extracted from fetal sheep liver and lung and subjected to SDS-PAGE and western blotting. Bound antibody was visualized using an HRP-based chemiluminescent detection system. For details, see Materials and methods. PiT-2 over-expressing MDTF cells were used as a positive control.
Expression pattern of PiT-2 protein in the liver during gestation
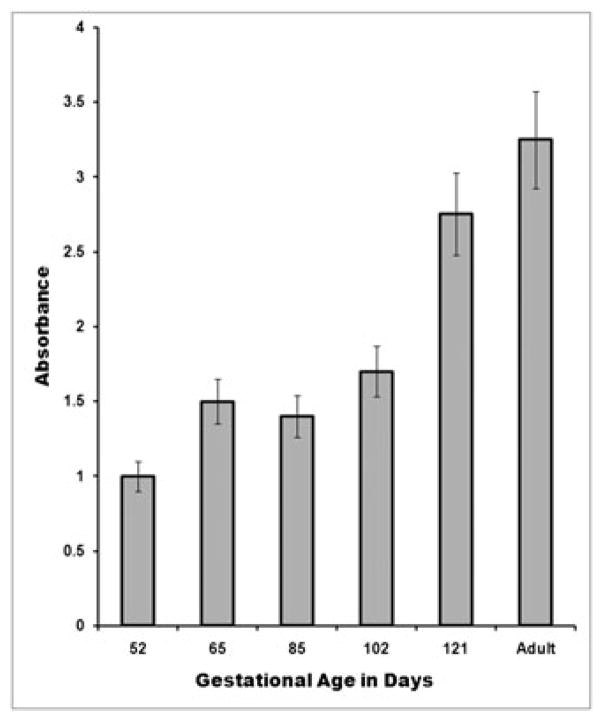
To directly test our hypothesis that the levels of PiT-2 protein expression vary as a function of gestational age and could thus provide an explanation for our previous observations [17], membrane proteins were extracted from the liver of fetal sheep at gestational ages of 52, 58–59, 65–66 and 102 days (term: 145 days). The levels of expression of the PiT-2 receptor in these samples were then quantified using a custom ELISA that we developed using the anti-PiT-2 antibody (for details, see Materials and methods). This analysis demonstrated (Figure 8) that the levels of PiT-2 protein expression were highest between gestational days 52–59, which correspond to the timeframe at which we began the analysis of the transduction efficiency in our previous study [17]. Subsequently, PiT-2 expression decreased as the gestational age advanced, dropping by approximately 40% at day 102 from the levels seen at days 52–59 of gestation. These findings thus correlated quite well with the results of our previous studies on transduction efficiency within the fetal liver after IUGT.
Figure 8.
Protein level expression of PiT-2 in fetal liver. Membrane proteins were extracted from liver of sheep fetuses of varying gestational ages, and the levels of PiT-2 protein quantified by ELISA, as detailed in the Materials and methods. PiT-2 over-expressing MDTF cells were used as a positive control; water and coating buffer were used as negative controls. Samples were run in triplicate. Error bars represent the SD.
Expression pattern of PiT-2 protein within the fetal lung
By contrast to the fetal liver, our previous findings within the fetal lung revealed that the transduction efficiency had increased with recipient age [17]. To assess whether these results could also be explained by alterations in the levels of expression of the PiT-2 receptor protein, we analyzed the lungs from fetal sheep at gestational ages in the range 45–120 days of gestation by ELISA. Similar to what we had observed in the liver, at the earliest time point of analysis (day 40), the levels of PiT-2 expression were at their lowest. Expression levels of PiT-2 then began to steadily rise as the gestational age increased, reaching their highest levels in the lung samples taken from postnatal animals (Figure 9), correlating well with the results of our previous studies on transduction efficiency. Overall, the results of these ELISA studies thus support the conclusion that the levels of expression of the PiT-2 protein vary as a function of gestational age and likely govern, at least in part, the efficiency of transduction within the fetal liver and lung after direct intraperitoneal injection of A-MRV vectors.
Figure 9.
Protein level expression of PiT-2 in fetal lung. Membrane proteins were extracted from lung of sheep fetuses of varying gestational ages, and the levels of PiT-2 protein quantified by ELISA, as detailed in the Materials and methods. PiT-2 over-expressing MDTF cells were used as a positive control; water and coating buffer were used as negative controls. Samples were run in triplicate. Error bars represent the SD.
Discussion
The present study attempted to elucidate the mechanism responsible for the alteration in transduction efficiency that we had previously observed as a function of recipient age after IUGT with murine retroviral-based vectors [17]. Importantly, these previous studies indicated that the maximal efficiency of transduction occurred at different times during gestation depending on the tissue examined, suggesting that some process within each individual tissue was responsible, rather than a generalized phenomenon as a result of fetal development. To unravel the mechanisms behind these observations, we began by examining the two factors that we considered the most likely primary determinants of retroviral transduction efficiency within the liver and lung after direct intra-peritoneal vector injection into the fetal sheep. Because the A-MRV vectors that we employed lack a protein to facilitate active nuclear transport, they can only transduce dividing cells. We thus reasoned that the proliferative status of the target cells would likely be one of the major factors determining the efficiency of retroviral transduction both in vitro and in vivo and, accordingly, began our analysis by quantitating proliferation within the liver and lung as a function of gestational age.
During development, the liver serves numerous important functions, requiring the large-scale proliferation of hepatocytes and other cells within the liver early in fetal life. It therefore stands to reason that the fetal liver should be more susceptible to transduction earlier in gestation with A-MRV retroviral vectors, which is exactly what we had seen in our previous studies. However, in the present study, we observed that the number of Ki67 + hepatocytes within the fetal liver progressively increased during the course of gestation, such that the percentage of proliferating hepatocytes more than doubled between 52 and 102 days of gestation. In addition to fetal hepatocytes, we also assessed the proliferative status of CD45+ hematopoietic cells and OV-6+ liver progenitors (oval cells) as a function of fetal age. The proliferation of hematopoietic cells within the fetal liver fluctuated in an apparently random fashion throughout gestation and did not correlate with transduction efficiency. With respect to oval cells, the absolute number of proliferating OV-6+ cells steadily increased as a function of gestational age, as seen with the hepatocytes. However, in contrast to the hepatocytes, the proliferative status of the oval cells appeared to correlate fairly well with their efficiency of transduction after IUGT. Thus, our analysis of the proliferative status of the major cell types within the fetal liver as a function of gestational age suggests that, although the proliferative rate may play a role in determining the efficiency of oval cell transduction, in the case of hepatocytes and hematopoietic cells, the proliferative rate is not the major factor contributing to an enhanced transduction efficiency at the early gestational ages observed in our previous studies.
Unlike the liver, the lungs only initiate significant expansion later in gestation with the formation of bronchioles and alveoli, in anticipation of having to fulfil the respiratory needs of the newborn upon parturition. Once again, this presumed proliferative status appeared to agree well with our previous findings indicating that the fetal lungs were more amenable to retroviral-mediated gene transfer later in gestation. However, our present quantitative analysis of the proliferative status within the fetal lung using Ki67 revealed that not only CK + epithelial cells, but also the majority of other cell types within the fetal lung were steadily dividing throughout gestation, irrespective of the developmental stage. Thus, similar to our results obtained with fetal liver, there was no apparent correlation between cell cycling and transduction efficiency within this tissue, leading us to conclude that the proliferative status of the cells within the tissues of the fetal recipient is not likely to be the main determinant of gene transfer efficiency. This lack of correlation between the proliferative status of the tissues and the observed transduction efficiency is in agreement with recent studies showing that, in contrast to the commonly held assumption, A-MRV do not require mitosis for transduction, as demonstrated by their ability to transduce terminally differentiated neuroendocrine cells and primary cortical neurones [21].
A-MRV vectors attach to and enter cells via binding to their cognate receptor, PiT-2, which is a transmembrane protein expressed in most cell types within the body that functions as a Na/Pi symporter. Given the lack of correlation between proliferation and observed transduction efficiency, we hypothesized that the expression level of PiT-2 was likely to be the major factor governing gene transfer after IUGT. In the present study, we examined the expression of PiT-2 at both mRNA and protein levels in the fetal liver and lung. The data obtained clearly demonstrate that PiT-2 expression levels (both mRNA and protein) vary within the liver and lung during fetal development. In both tissues, the expression level of PiT-2 correlated quite well with the pattern of A-MRV transduction efficiency that we observed in our previous studies, supporting the conclusion that PiT-2 expression levels are one of the main factors governing the efficiency of IUGT, at least within the two tissues we examined. Immunofluorescence would need to be performed using antibodies to both PiT-2 and NPTII to confirm that the PiT-2 levels are directly responsible for the enhanced levels of transduction observed during certain periods of gestation. Unfortunately, the antibody that we utilized in the present study does not allow us to distinguish between alterations in the low, endogenous levels of expression of PiT-2 with respect to whole cells or tissue sections. Moreover, the one commercially available antibody to human PiT-2 (Santa Cruz Biotechnology) fails to cross-react with the sheep homolog, precluding its use in our studies. Therefore, the data reported in the present study is correlative because definitive proof that PiT-2 levels determine the efficiency of transduction within the tissues of the developing fetal sheep will need to await the development of more robust, broader specificity antibodies to this protein.
To our knowledge, the present study is the first to examine the expression of PiT-2 in a large animal model, and is also the first to report on the apparent developmental regulation of this important symporter/retroviral receptor. Our findings showing that the expression levels of the PiT-2 receptor can explain, at least in part, the observed gestational age-related differences in transduction efficiency after A-MRV-mediated IUGT suggest that it may ultimately be possible to target specific organs/cell types after IUGT by administering the vector at a time when PiT-2 expression is maximal within the desired target tissue. This would not only concentrate the therapeutic effect within the desired cell types, but also would minimize the transduction of undesirable nontarget cell types within the developing fetus, such as the cells of the germline [22,23].
Acknowledgments
This work was supported by grant #HD43038 from the National Institutes of Health.
References
- 1.Porada CD, Tran N, Eglitis M, et al. In utero gene therapy: transfer and long-term expression of the bacterial neo(r) gene in sheep after direct injection of retroviral vectors into preimmune fetuses. Hum Gene Ther. 1998;9:1571–1585. doi: 10.1089/hum.1998.9.11-1571. [DOI] [PubMed] [Google Scholar]
- 2.Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669–672. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- 3.Suzuki Y, Craigie R. The road to chromatin –nuclear entry of retroviruses. Nat Rev Microbiol. 2007;5:187–196. doi: 10.1038/nrmicro1579. [DOI] [PubMed] [Google Scholar]
- 4.Barrette S, Douglas J, Orlic D, et al. Superior transduction of mouse hematopoietic stem cells with 10A1 and VSV-G pseudotyped retrovirus vectors. Mol Ther. 2000;1:330–338. doi: 10.1006/mthe.2000.0052. [DOI] [PubMed] [Google Scholar]
- 5.Kohn DB, Weinberg KI, Nolta JA, et al. Engraftment of gene-modified umbilical cord blood cells in neonates with adenosine deaminase deficiency. Nat Med. 1995;1:1017–1023. doi: 10.1038/nm1095-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunbar CE, Cottler-Fox M, O’Shaughnessy JA, et al. Retrovirally marked CD34-enriched peripheral blood and bone marrow cells contribute to long-term engraftment after autologous transplantation. Blood. 1995;85:3048–3057. [PubMed] [Google Scholar]
- 7.Bodine DM, Moritz T, Donahue RE, et al. Long-term in vivo expression of a murine adenosine deaminase gene in rhesus monkey hematopoietic cells of multiple lineages after retroviral mediated gene transfer into CD34+ bone marrow cells. Blood. 1993;82:1975–80. [PubMed] [Google Scholar]
- 8.Kiem HP, Heyward S, Winkler A, et al. Gene transfer into marrow repopulating cells: comparison between amphotropic and gibbon ape leukemia virus pseudo-typed retroviral vectors in a competitive repopulation assay in baboons. Blood. 1997;90:4638–4645. [PubMed] [Google Scholar]
- 9.Kavanaugh MP, Miller DG, Zhang W, et al. Cell-surface receptors for gibbon ape leukemia virus and amphotropic murine retrovirus are inducible sodium-dependent phosphate symporters. Proc Natl Acad Sci USA. 1994;91:7071–7075. doi: 10.1073/pnas.91.15.7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Virkki LV, Biber J, Murer H, et al. Phosphate transporters: a tale of two solute carrier families. Am J Physiol Renal Physiol. 2007;293:F643–F654. doi: 10.1152/ajprenal.00228.2007. [DOI] [PubMed] [Google Scholar]
- 11.Wilson CA, Eiden MV, Anderson WB, et al. The dual-function hamster receptor for amphotropic murine leukemia virus (MuLV), 10A1 MuLV, and gibbon ape leukemia virus is a phosphate symporter. J Virol. 1995;69:534–537. doi: 10.1128/jvi.69.1.534-537.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Overbaugh J, Miller AD, Eiden MV. Receptors and entry cofactors for retroviruses include single and multiple transmembrane-spanning proteins as well as newly described glycophosphati-dylinositol-anchored and secreted proteins. Microbiol Mol Biol Rev. 2001;65:371–389. doi: 10.1128/MMBR.65.3.371-389.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chien ML, Foster JL, Douglas JL, et al. The amphotropic murine leukemia virus receptor gene encodes a 71-kilodalton protein that is induced by phosphate depletion. J Virol. 1997;71:4564–4570. doi: 10.1128/jvi.71.6.4564-4570.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller DG, Miller AD. Inhibitors of retrovirus infection are secreted by several hamster cell lines and are also present in hamster sera. J Virol. 1993;67:5346–5352. doi: 10.1128/jvi.67.9.5346-5352.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson CA, Eiden MV. Viral and cellular factors governing hamster cell infection by murine and gibbon ape leukemia viruses. J Virol. 1991;65:5975–5982. doi: 10.1128/jvi.65.11.5975-5982.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boyer CJ, Baines AD, Beaulieu E, et al. Immunodetection of a type III sodium-dependent phosphate cotransporter in tissues and OK cells. Biochim Biophys Acta. 1998;1368:73–83. doi: 10.1016/s0005-2736(97)00159-4. [DOI] [PubMed] [Google Scholar]
- 17.Porada CD, Park PJ, Almeida-Porada G, et al. Gestational age of recipient determines pattern and level of transgene expression following in utero retroviral gene transfer. Mol Ther. 2005;11:284–293. doi: 10.1016/j.ymthe.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Jobbagy Z, Olah Z, Petrovics G, et al. Up-regulation of the Pit-2 phosphate transporter/retrovirus receptor by protein kinase C epsilon. J Biol Chem. 1999;274:7067–7071. doi: 10.1074/jbc.274.11.7067. [DOI] [PubMed] [Google Scholar]
- 19.Jobbagy Z, Garfield S, Baptiste L, et al. Subcellular redistribution of Pit-2 P(i) transporter/amphotropic leukemia virus (A-MuLV) receptor in A-MuLV-infected NIH 3T3 fibroblasts: involvement in superinfection interference. J Virol. 2000;74:2847–2854. doi: 10.1128/jvi.74.6.2847-2854.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feldman SA, Farrell KB, Murthy RK, et al. Identification of an extracellular domain within the human PiT2 receptor that is required for amphotropic murine leukemia virus binding. J Virol. 2004;78:595–602. doi: 10.1128/JVI.78.2.595-602.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu XH, Xu W, Russ J, et al. The host range of gammaretroviruses and gammaretroviral vectors includes post-mitotic neural cells. PLoS One. 2011;6:e18072. doi: 10.1371/journal.pone.0018072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park PJ, Colletti E, Ozturk F, et al. Factors determining the risk of inadvertent retroviral transduction of male germ cells after in utero gene transfer in sheep. Hum Gene Ther. 2009;11(3):201–215. doi: 10.1089/hum.2007.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porada CD, Park PJ, Almeida-Porada G, et al. Gestational age of recipient determines pattern and level of transgene expression following in utero retroviral gene transfer. Mol Ther. 2005;(112):284–293. doi: 10.1016/j.ymthe.2004.09.009. [DOI] [PubMed] [Google Scholar]