Abstract
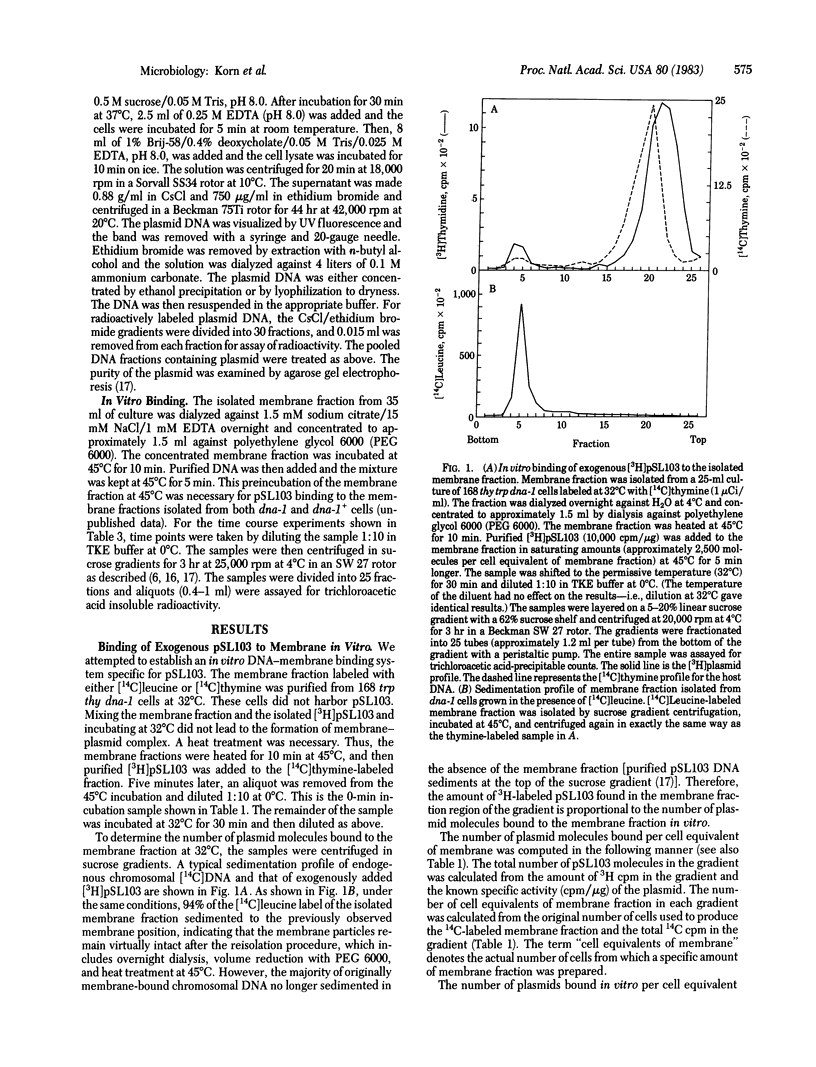
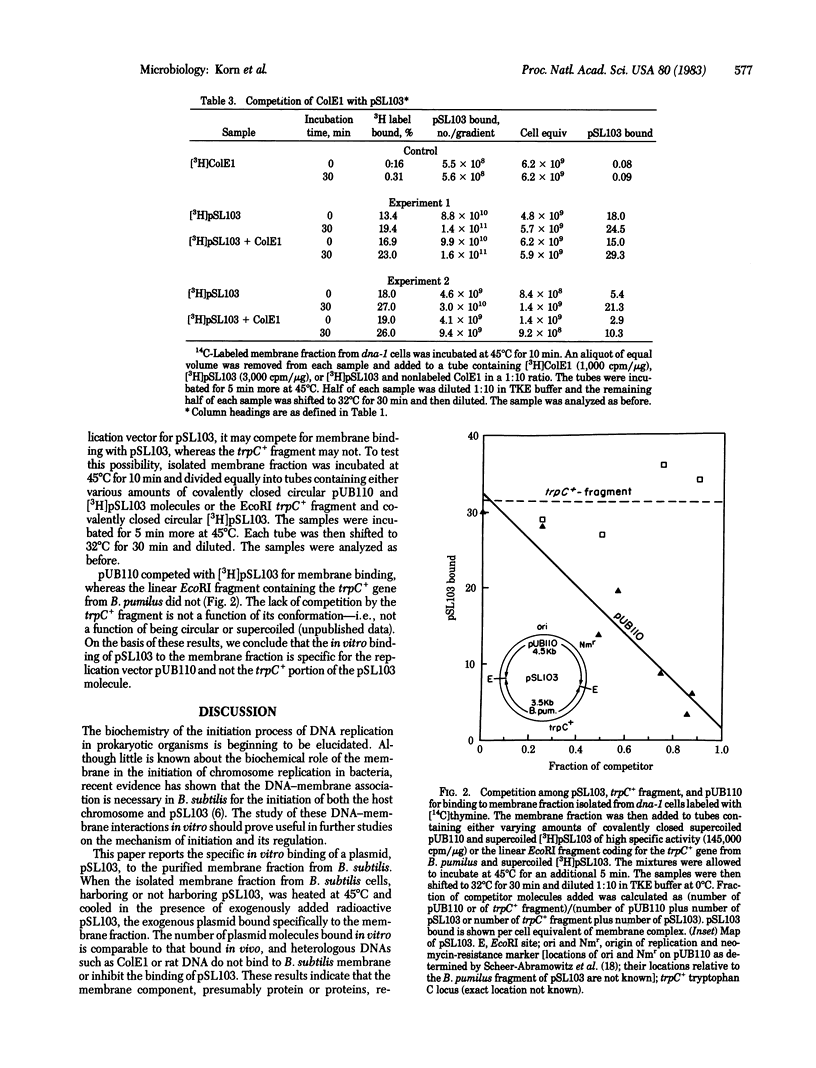
A model system has been developed to study the in vitro binding of a plasmid to the membrane fraction from Bacillus subtilis. The plasmid DNA molecule used in these studies was pSL103 (8.0 kilobases), a chimeric plasmid consisting of a Staphylococcus aureus plasmid (pUB110, 4.5 kilobases) and a DNA fragment (3.5 kilobases) from Bacillus pumilus carrying trpC+ gene. This plasmid replicates in B. subtilis cells, and its in vivo membrane binding (as well as its replication) is dependent on the product of a DNA initiation gene, dna-1, of B. subtilis. In this paper we demonstrate the in vitro specific binding of exogenous pSL103 to the isolated membrane fraction. This in vitro binding is specific to the origin-containing portion (pUB110) of pSL103. The trpC+-carrying portion neither binds to the membrane fraction nor competes with pSL103 for binding to the membrane fraction in vitro. ColE1 plasmid, which does not replicate in B. subtilis, neither binds to the B. subtilis membrane fraction nor competes with pSL103 for binding.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostopoulos C., Spizizen J. REQUIREMENTS FOR TRANSFORMATION IN BACILLUS SUBTILIS. J Bacteriol. 1961 May;81(5):741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fielding P., Fox C. F. Evidence for stable attachment of DNA to membrane at the replication origin of Escherichia coli. Biochem Biophys Res Commun. 1970 Oct 9;41(1):157–162. doi: 10.1016/0006-291x(70)90482-1. [DOI] [PubMed] [Google Scholar]
- Imada S., Carroll L. E., Sueoka N. Genetic mapping of a group of temperature-sensitive dna initiation mutants in Bacillus subtilis. Genetics. 1980 Apr;94(4):809–823. doi: 10.1093/genetics/94.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamata D., Gross J. D. Isolation and genetic analysis of temperature-sensitive mutants of B. subtilis defective in DNA synthesis. Mol Gen Genet. 1970;108(3):277–287. doi: 10.1007/BF00283358. [DOI] [PubMed] [Google Scholar]
- Keggins K. M., Lovett P. S., Duvall E. J. Molecular cloning of genetically active fragments of Bacillus DNA in Bacillus subtilis and properties of the vector plasmid pUB110. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1423–1427. doi: 10.1073/pnas.75.3.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Sullivan A., Sueoka N. Sequential replication of the Bacillus subtilis chromosome. IV. Genetic mapping by density transfer experiment. J Mol Biol. 1967 Jul 28;27(2):349–368. doi: 10.1016/0022-2836(67)90025-3. [DOI] [PubMed] [Google Scholar]
- O'Sullivan M. A., Sueoka N. Membrane attachment of the replication origins of a multifork (dichotomous) chromosome in Bacillus subtilis. J Mol Biol. 1972 Aug 21;69(2):237–248. doi: 10.1016/0022-2836(72)90228-8. [DOI] [PubMed] [Google Scholar]
- Parker D. L., Glaser D. A. Chromosomal sites of DNA-membrane attachment in Escherichia coli. J Mol Biol. 1974 Aug 5;87(2):153–168. doi: 10.1016/0022-2836(74)90140-5. [DOI] [PubMed] [Google Scholar]
- Scheer-Abramowitz J., Gryczan T. J., Dubnau D. Origin and mode of replication of plasmids pE194 and pUB110. Plasmid. 1981 Jul;6(1):67–77. doi: 10.1016/0147-619x(81)90054-8. [DOI] [PubMed] [Google Scholar]
- Shivakumar A. G., Dubnau D. Differential effect of hydroxyurea on the replication of plasmid and chromosomal DNA in Bacillus subtilis. J Bacteriol. 1978 Dec;136(3):1205–1207. doi: 10.1128/jb.136.3.1205-1207.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder R. W., Young F. E. Association between the chromosome and the cytoplasmic membrane in Bacillus subtilis. Biochem Biophys Res Commun. 1969 May 8;35(3):354–362. doi: 10.1016/0006-291x(69)90506-3. [DOI] [PubMed] [Google Scholar]
- Sueoka N., Hammers J. M. Isolation of DNA-membrane complex in Bacillus subtilis. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4787–4791. doi: 10.1073/pnas.71.12.4787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueoka N., Quinn W. G. Membrane attachment of the chromosome replication origin in Bacillus subtilis. Cold Spring Harb Symp Quant Biol. 1968;33:695–705. doi: 10.1101/sqb.1968.033.01.078. [DOI] [PubMed] [Google Scholar]
- White K., Sueoka N. Temperature-sensitive DNA synthesis mutants of Bacillus subtilis--appendix: theory of density transfer for symmetric chromosome replication. Genetics. 1973 Feb;73(2):185–214. doi: 10.1093/genetics/73.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston S., Sueoka N. DNA-membrane association is necessary for initiation of chromosomal and plasmid replication in Bacillus subtilis. Proc Natl Acad Sci U S A. 1980 May;77(5):2834–2838. doi: 10.1073/pnas.77.5.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston S., Sueoka N. Membrane association of a Staphylococcus aureus plasmid in Bacillus subtilis. J Bacteriol. 1980 Apr;142(1):339–343. doi: 10.1128/jb.142.1.339-343.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K., Yoshikawa H. Topography of chromosome membrane junction in Bacillus subtilis. Nat New Biol. 1973 Aug 15;244(137):204–206. doi: 10.1038/newbio244204a0. [DOI] [PubMed] [Google Scholar]
- Yoshikawa H., Yamaguchi K., Seiki M., Ogasawara N., Toyoda H. Organization of the replication-origin region of the Bacillus subtilis chromosome. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):569–576. doi: 10.1101/sqb.1979.043.01.063. [DOI] [PubMed] [Google Scholar]


