Table 1.
Initial Studies towards α-Amination of Carbonyls
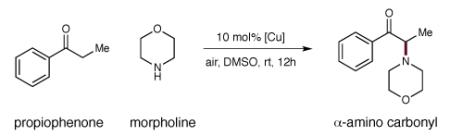
| entry | [Cu] catalyst | solvent | yielda |
|---|---|---|---|
| 1 | CuBr2 | MeCN | 68% |
| 2 | CuCl2 | MeCN | 2% |
| 3 | CuBr | MeCN | 31% |
| 4 | Cu(TFA)2 | MeCN | 0% |
| 5b | Cu(TFA)2 | MeCN | 50% |
| 6c | CuBr2 | MeCN | 62% |
| 7 | CuBr2 | CHCl3/EtOAc | 45% |
| 8 | CuBr2 | THF | 67% |
| 9 | CuBr2 | DMF | 71% |
| 10 | CuBr2 | DMSO | 93%d |
GC yield using Bn2O as an internal standard.
With 30 mol% LiBr.
Performed over 24 hours.
Isolated Yield.
