Abstract
Purpose of the review
New research supports the intuitive observation that many persons classified as obese are healthy, and should not treated and categorized medically as diseased. There is increasing agreement that major blood biomarkers are often not discriminatory, as for example, the return to normal blood glucose levels in bariatric patients who do not have long terms benefits. Although weight loss is appreciated to improve metabolic and inflammatory parameters, the cellular and immune factors that couple obesity to cardiometabolic risk are only partially understood.
Recent findings
Reduced body mass index upon successful bariatric surgery does not always result in reduced pericardial fat; certain patients gain ectopic fat, which should be considered an adverse response. There is emerging evidence that pericardial fat volume and brown fat stores may provide individualized patient assessments.
Summary
Some obese persons can be relieved of the additional stigma of classification in a major disease category and unnecessary medical interventions and costs can be reduced. Other patients should be monitored more closely for unexpected adverse outcomes.
Keywords: obesity, inflammation, insulin resistance, bariatric surgery, pericardial fat, metabolically healthy obese, MRI
Introduction
Diet-induced obesity and its metabolic complications pose a serious and worsening problem for public health worldwide [1]. If incidence trends continue unabated, the prevalence of cardiovascular disease (CVD) and Type 2 diabetes (T2D) arising as co-morbidities of obesity is predicted to increase dramatically. These developments will define a medical disaster of historic proportions that will affect all regions of the world, ethnic groups and socioeconomic classes. However, as will be described below, these risks are distributed unevenly. There is therefore great urgency to identify populations at greatest risk for the complications of obesity in order to focus scientific effort and tailor therapeutic interventions.
In humans and mouse models of human metabolism, weight gain induced by the consumption of a hypercaloric diet without compensatory increases in physical activity leads to deteriorated metabolic health. This decline usually features increased insulin resistance, glucose intolerance, dyslipidemia, hypercholesterolemia and other metabolic and serological abnormalities that are strongly coupled with increased risk for CVD and T2D. A critical feature of these developments that has been receiving much attention recently is the pro-inflammatory alteration of immune cell subsets in peripheral blood as well as within insulin-resistant adipose tissue. Specifically, analysis of the blood of many insulin-resistant, obese patients (body mass index (BMI) ≥ 30 kg/m2) reveals significant elevations of pro-inflammatory cytokines (e.g., tumor necrosis factor-α, interleukin-6), declines in cardioprotective adipokines (e.g. adiponectin) and other cytokines in addition to elevated fasting glucose and insulin. These patients also exhibit local infiltration of adipose tissue with pro-inflammatory macrophages, pro-inflammatory T cells and a corresponding decline in these same tissues in numbers of alternatively activated macrophages and anti-inflammatory regulatory T cells [2]. Chronic, unresolved inflammation of this type that accompanies diet-induced obesity is thought to be a critical factor in progression to T2D [3•].
In addition, the incidence of obesity-associated cancers is increasing [4,5,6] and is expected to track with the obesity epidemic, but with lagging kinetics commonly seen in exposure-associated cancers. There is evidence to support the hypothesis that the chronic inflammatory states in insulin-resistant obesity provide over years an important contribution to primary carcinogenesis or angiogenesis and metastasis of already extant, cryptic tumors [7,8•]. Along with tobacco-associated risks, obesity is now considered a highly dangerous risk factor for specific cancers [9]. The most serious of these tumors are colon and renal cancers in men and women, breast cancer in post-menopausal women and endometrial cancer. Alarmingly, obesity-related cancer is now thought to contribute to 14% of male and 20% of female cancer mortality in the United States [4]. The specific, cancer-related roles for increased Th17 cells, reduced T regulatory cells, pro-inflammatory macrophages and other adipose tissue-infiltrating subtypes of immune cells [2], and their cancer-related crosstalk with metabolism in obesity, is very poorly understood.
Weight loss through increased energy expenditure, such as through increased aerobic exercise, or through reduced caloric intake, have been demonstrated to produce desired improvements in metabolic and inflammatory parameters. Many therapeutic strategies for obesity such as diet-induced weight loss, and Roux-en-y gastric bypass surgery (RYGB), have targeted the reduction of abdominal fat, also termed ‘visceral’ or ‘central’ adipose tissue, although total body fat loss also occurs. Diet-induced weight loss can, in the short-term, improve the metabolic complications of central obesity; however, most obese persons using this strategy regain their lost weight over time [10]. Indeed, a significant fraction of bariatric patients (20.4% for morbidly obese patients (BMI ≥ 40 kg/m2) and 34.9% for super obese patients in one study) [11] regain significant weight in the two to three years after surgery, unrelated to surgical failure. The cellular, molecular and epigenetic mechanisms that underlie these problems are currently unknown. For many morbidly obese patients, RYGB remains a popular choice: bariatric surgeries in the United States increased from 13,000 in 1990 to 100,000 in 2003 [12] and 200,000 in 2006. The popularity of RYGB is chiefly due to the magnitude of the weight loss achieved is higher (≥ 30%) compared to diet-induced weight loss (5–10%). Furthermore, weight loss for most bariatric subjects is sustained beyond the 6–12 months generally achieved by dietary weight loss. After RYGB, most subjects show a dramatic improvement and even resolution of comorbidities such as T2D, sleep apnea, and dyslipidemia, often prompting withdrawal of medications shortly after surgery [13]. Bariatric surgery results in better glucose control than medical therapies; however, the BMI prior to surgery and the extent of weight loss following surgery do not necessarily predict the improvement in hyperglycemia [14•]. In fact, comorbidities do not resolve in all subjects and factors that can predict the difference in outcomes between these patients and the former are sought to aid clinicians in making prognoses and treatment plans.
BMI alone appears to be only weakly associated with certain obesity-related diseases. In view of the considerable individual-level variation in BMI and metabolic responses to bariatric surgery, some investigators have begun to wonder whether genetic or epigenetic predisposition to weight regain [15] is a widespread and common phenotype. A groundbreaking study recently demonstrated that adverse responses in cardiometabolic parameters occur in response to regular exercise in about 10% of the general population, but the mechanism is unknown [16••]. This observation that simple, regular, cardiovascular exercise, heretofore generally considered to be harmless and of significant cardiometabolic benefit, should actually be harmful to certain individuals not thought to be at risk, raises clear and novel concern that bariatric surgery may also be harmful to certain obese patients, and moots an urgent question: can we identify such surgical candidates in advance, in order to direct them to alternative weight loss interventions?
Subpopulations of obese and unhealthy persons
Obesity was defined as a disease by the American Medical Association in June 2013, in welcome recognition of the strong epidemiological association between BMI and numerous serious conditions, yet the correlation is not perfect; mechanisms that link elevated BMI to specific disease risks are still not fully understood.
Metabolically healthy obese (MHO) and metabolically unhealthy lean (MUL) subjects
Obese persons manifest a spectrum of complications as described above, including elevated risks for T2D, CVD, certain cancers, stroke and musculoskeletal diseases. However, it has been appreciated for many years that about a quarter of the adult obese population exhibits notably fewer of these complications and do not meet all the criteria for metabolic syndrome (MS) [17]. Certain of these individuals at the ‘healthy end’ of the spectrum of obesity complications lack systemic and local features of chronic, unresolved inflammation that are normally found in humans as well as animal models of diet-induced obesity [18,19,20]; this lower inflammatory profile associates with significantly reduced cardiometabolic risk [21,22]. These so-called ‘metabolically healthy’ obese subjects [23] are interesting because they appear to bend the rules of metabolism [24], and demonstrate insulin-sensitive obesity, including a robust ability to clear infused glucose in a hyperinsulinemic-euglycemic clamp measurement. Critical opinion has held that these individuals do not enjoy a stable state of ongoing metabolic protection, but are merely passing through a period of uncertain duration subclinical to the definition of MS, in which some of the cardiometabolic risk factors are present. Thus, according to this view, all ‘metabolically healthy’ obese individuals are merely delayed on a well-trodden path to inevitable, overt MS. On the other hand, this view fails to account for some subjects who have extremely high BMI but persistently normal glucose infusion rates [24].
Certain other individuals also fail to show the expected associations between BMI and metabolism, such as the ‘metabolically unhealthy lean’, also termed ‘metabolically obese normal weight’ subjects [25,26]. These individuals are lean with respect to BMI, but show many of the serological features of insulin resistant obesity, including hyperinsulinemia, hypertriglyceridemia, hypoadiponectinemia and elevated risk for CVD and T2D. Clinical investigation of these subjects suggests that for some individuals, ectopic adipose tissue [27], particularly in visceral [28] or pericardial depots of adipose tissue, may be a major factor that compromises metabolic health. These depots need not be large to promote cardiometabolic risk, and thus BMI may be a relatively insensitive measure to detect these depots. Thus, BMI is poorly associated with metabolic health.
Long term stability of the MHO and MUL phenotypes
An important question presents itself: are the MHO and MUL metabolic and adipose depot profiles defined by genetics, by diet and exercise or by stage in lifespan? Critical questions of clinical significance are: are these phenotypes interconvertible, and can a patient in an unhealthy category improve their health by appropriate lifestyle changes, bariatric surgery or drugs? Or are these phenotypes relatively refractory to clinical management, because patients will tend to revert to type once an intervention is made? Most seriously, are there unanticipated consequences of our therapies that leave patients less well off than their basal metabolic status when they presented for treatment?
Only two studies have addressed the question of long-term stability of MHO subtype, and both of these were in Asian populations. Bradshaw and colleagues [29•] investigated MHO subjects in an African American cohort recruited in 1987 – 1989 from the Atherosclerosis Risk in Communities (ARIC) Study. Over 9 years of follow-up, the authors observed that body size was positively associated with emergent MS, whereas physical activity was negatively associated. There is evidence that the MHO subtype is transitory in some patients, a plateau on the way to MS, but with slower kinetics than unhealthier patients. Interestingly, these authors report a positive association between MS and age and an inverse association with alcohol intake. Some of the reported differences could arise from the definition of MS. Many MHO subjects have some of the features of MS but are still classified as not having MS. Commentary from Lopez-Miranda and Perez-Martinez has called for the careful definition of MUL individuals. There is disagreement in the field about nature and severity of elevated risks that may be experienced by MHO and MUL subjects.
An older study by Miller and colleagues [30], who investigated MS risks among retired football players in the United States National Football League (NFL), reported that the prevalence of MS was higher among linemen than among non-linemen. The physical differences between the body mass and composition of these two types of NFL players can be appreciated by the need for weight and strength among line backers, but the need for agility and speed among quarterbacks. Upon retirement from the league and cessation of intense physical training, linemen were at almost double the risk of MS than their teammates with lower BMIs. Similar patterns have been observed for Sumo wrestlers [31]. These individuals consume a calorie-dense diet but engage intense, daily physical training. Despite extremely high BMI measurements in some cases, these wrestlers maintain healthy metabolism, including normal blood glucose and lipid values, and relatively low visceral fat burden. Upon retirement, Sumo wrestlers are at risk for rapid onset of MS, CVD and T2D if the regimen of exercise is not maintained.
Finally, Karelis and colleagues [32] had reported that a 6-month calorie restricted diet actually reduced insulin sensitivity in MHO females, mean age (57.7 ±4.5) years, as measured by hyperinsulinemic euglycemic clamp, whereas insensitivity improved in matched metabolically unhealthy subjects, although both groups lost weight. This result suggested that weight loss is not an appropriate standard of care for all subtypes of obese patients, although how the mechanisms differ between subtypes is unknown. Janiszewski and Ross [33] re-explored the consequences of weight loss among MHO men and women (mean age 61.4 ±11.8 and 61.1 ±12.0 years, respectively), using a variety of methods in a 3-month program to reduce BMI, including diet or exercise (either aerobic exercise or resistance training plus aerobics). They found that both metabolically healthy and unhealthy subjects improved insulin sensitivity, in disagreement with Karelis and colleagues. It is possible that uncontrolled variables explain the discrepancy, such as baseline inflammatory status, or genetic/epigenetic variation in genes important for metabolism. It remains unclear whether MHO status reflects metabolism, inflammation or both, nor is it yet clear whether these two factors can vary in isolation from the other.
Variance in responses among RYGB patients
It is becoming increasingly clear that smaller, ectopic depots are directly related to T2D by locally or systemically-mediated effects [34], and reductions in these depots may improve organ function. Several fat depots bear investigation, particularly intrahepatic fat and pericardial fat. In the case of the former, the presence of T2D increases the morbidity associated with non-alcoholic steatohepatitis (NASH) regardless of BMI [35,36]. Whereas an estimated 10–24% of the general population is affected, the prevalence among obese persons is greatly (4–6 fold) increased [37,38,39]. In the case of pericardial fat, this depot surrounds the heart and encompasses layers under (epicardial) and over (pericardial) the visceral layer of the epicardium; in the literature, the terms are often used interchangeably although they are not identical adipose tissue [40]. Both pericardial fat regions are metabolically active and secrete several bioactive proteins, including adiponectin, resistin, various inflammatory molecules, and is a rich source of free fatty acids (FFA) [41,42,43,44]. Pericardial fat could therefore exert local effects by multiple mechanisms on the underlying anatomic structures, including the heart and coronary arteries.
Recent findings
Magnetic Resonance Imaging (MRI) measurement of ectopic fat depots
Recent studies of humans using a variety of approaches have suggested that, rather than overall obesity, regional fat distribution plays an important role in cardiac modification [45,46,47,48]. Accumulating evidence indicates that the quantity of pericardial lipid volume is an important indicator that can stratify vascular and metabolic risk. In asymptomatic individuals without MS, higher pericardial lipid is found in subjects with higher fasting glucose levels and may even predicts the development of MS [49,50]. Reduction in pericardial lipid volume correlates to improvement in insulin resistance [51] in obese individuals [52].
Among various regional fat depots, pericardial fat is of particular interest and has been proposed as a superior indicator of cardiac function because of the anatomic vicinity. Iacobellis and colleagues [53] studied 30 obese subjects and compared with 20 lean control volunteers using 2D echocardiography. They found an increase in the epicardial fat thickness strongly correlated with the decrease of left ventricular (LV) diastolic function. Ruberg and colleagues [49] reported negative correlations between pericardial fat and LV cardiac output/stroke volume in an obese group with MS. Both studies suggested local interactions between the regional adipose tissue and LV function, but without ruling out the influence from systemic effects caused by overall obesity. It became particularly interesting when Fox and colleagues performed a large population study [54] in 2009. They reported that pericardial fat correlated with LV end-diastolic volume and atrial dimension, but these correlations did not hold after the multivariable adjustment for overall adiposity, and suggested that systemic influences might override local effects. This study [54] did not examine the specific LV function as did the previous two [49,53]. However, the same rationale might apply, which is the previously observed relations between the local fat and cardiac function [49,53] may actually be the carryover effect from overall obesity.
Most but not all RYGB subjects lose pericardial fat
We hypothesized that certain patients would show adverse changes in these smaller ectopic depots in response to RYGB. To test this hypothesis, we conducted a pilot study of five subjects undergoing bariatric surgery. We obtained MRI of the fat surrounding the heart before and 10–12 months after surgery. These pericardial fat depots are clearly not amenable to quantification by invasive procedures, but can be seen and quantified by non-invasive imaging methods, especially MRI, computed tomography (CT) and 2D echo. Whereas cardiac CT and 2D echo can distinguish the epicardium, this is more difficult in MR images. All subjects lost 20–40% of total body mass 10–12 months after surgery. However, the quantity of pericardial fat lost was not predicted by the quantity of total fat loss. One individual even showed a gain of pericardial fat. This result dramatically illustrates the need for quantitative MRI for each individual, and presents the opportunity for discovery of a biomarker that can distinguish responders and non-responders. Conversely, other studies have shown that weight loss results in benefits greater than predicted by their attained BMI, but the mechanisms are not understood. Our approaches in this study could shed insight into why some subjects are better responders to RYGB. Similar losses of pericardial fat can be achieved via weight loss induced by lifestyle intervention, suggesting that weight loss itself mediates these changes.
Our recent study of pericardial fat and cardiac function highlighted individual differences very dramatically [49]. We studied obese subjects with MS but without known atherosclerotic disease and healthy controls with MRI to quantify pericardial and periaortic lipid volumes, and cardiac function. Pericardial lipid negatively correlated to cardiac output and stroke volume, thus indicating an adverse effect of fat on these functions. Pericardial, intrahepatic and periaortic lipid volumes were increased in obese subjects vs. controls and were strongly and positively correlated. However, the fat volumes were independent of BMI among obese subjects. In conclusion, ectopic storage of lipid in anatomically distinct depots appeared tightly correlated but independent of body size. These findings underscore the utility of MRI to assess individual differences in ectopic lipid that are not predictable from BMI. The dissociation of the quantity of pericardial fat and BMI reported [49] held true when we refined the analysis of total pericardial fat by quantifying fat surrounding the different regions of the heart. As in our previous study [49], in this study group with more participants, the lack of association between local fat and overall obesity (BMI) was also observed with statistical significance using Pearson correlation.
Measurement of pericardial fat in obese and T2D persons can provide a means to find healthy persons within a large population that is considered uniformly unhealthy. These persons can be treated differently, both psychologically (reducing the stigma of their “disease”) and with drug therapies. They are more likely to have better cardiac function and a lower long term risk for CVD. We have proposed that quantification pericardial fat, which can be achieved with non-invasive MRI and other imaging modalities, will provide an assessment that is more stable than blood biomarkers and blood pressure. The amount of fat will not fluctuate significantly over short time periods, and can provide a window to look for other markers of MHO status.
Metabolic syndrome can also be present in subjects without obesity [55•]; increased cardiometabolic risk may be present in individuals with non-obese BMI values [56•]. MR images obtained in a non-obese subject as illustrated by an axial slice at the level of the heart showed very little subcutaneous fat compared to our subjects previously reported [49], but revealed prominent pericardial and periaortic fat (Figure 1). In this normal BMI subject, extensive irregular atherosclerotic thickening of the descending thoracic aorta is also noted. This observation supports the overall hypothesis that metabolism and pericardial fat must be considered along with BMI before classifying a patient as healthy or unhealthy and in need of treatment.
Figure 1.
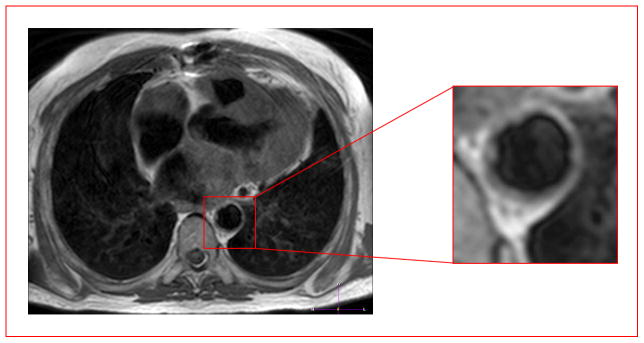
Pericardial fat can be expanded in otherwise lean but metabolically unhealthy subjects. MRI images of a metabolically unhealthy, lean (MUL) subject, showing ectopic fat deposition consistent with previous reports of ectopic adipose tissue accumulation [27,28] in such subjects. An axial T1-weighted black-blood image obtained through the thorax shows pericardial fat (bright regions surrounding the heart) and aortic atherosclerosis (shown in the red box enlargement) (irregular thickening of the vessel wall) in a non-obese subject with metabolic syndrome. There is also prominent fat surrounding the coronary above the aorta.
Conclusions
The healthy human heart contains little fat, but fat can accumulate to cover both the heart muscle and the coronary arteries. The more localized depot around arteries is associated with the severity of coronary atherosclerotic lesions in both non-obese and obese people [57], possibly because of direct secretion of fatty acids and inflammatory mediators such as IL-1β, IL-6, MCP-1 and TNF-α [58]. Excess fat can also be found in droplets within muscle cells (intramyocellular fat), a depot that is detected by MR spectroscopy rather than MR imaging. The characteristic volumes together with immunometabolic properties have not been studied in MHO and MUL individuals. All these fat depots may be useful markers to evaluate patients who are candidates for RYGB treatment as well as for their responses and adverse events. Our studies and results from others provide sufficient but not conclusive data to support the hypothesis that MHO subjects have significantly less pericardial fat proportional to BMI than obese subjects with MS, whereas MUL subjects have more pericardial fat proportional to BMI than lean, healthy subjects. The volume of pericardial fat as assessed by non-invasive imaging, the ratio of BMI to pericardial fat, or the metabolic activity of pericardial fat, may prove useful as noninvasive measurements that combine well with blood cytokine and adipokine concentrations, to stratify risk for obesity-associated cancers.
Our overall conclusion is that not all obesity carries the same cardiometabolic risk and that BMI is an insufficient clinical parameter to make well-informed medical decisions for optimum treatment in obesity. Markers such as pericardial fat, which is the most easily imaged heart fat depot, has a known association with cardiovascular risk and may be a direct causative agent, must be explored more extensively. MRI, which can be safely repeated in the same subject over time, could provide a clinical exam to stratify patients for their subsequent therapies.
Key points that summarize this report.
The American Medical Association (AMA) has only just classified obesity as a disease (on 18th June 2013), a determination that overturned the recommendation of the AMA’s own committee charged with study of this question; the issue has been controversial in part because of a widespread misconception that obesity is a lifestyle choice, but also because the link between elevated BMI and life threatening diseases is not perfect.
We are in agreement with other investigators that certain obese individuals appear to be protected from some of the expected cardiometabolic complications of obesity, while other individuals who are lean show surprisingly elevated risks for these same complications.
We propose that deeper investigation of these ‘off-diagonal’ populations will reveal important mechanisms that couple adipose tissue distribution and function to whole-body immunometabolism and health risks.
We hypothesize that these mechanisms likely involve small depots of ectopic fat, in particular pericardial fat, that are metabolically active, likely inflamed and critically contribute to elevated risks of cardiometabolic diseases and obesity-associated cancers, without contributing significantly to BMI.
Acknowledgments
We thank Caroline Apovian, Ning Hua, Frederick Ruberg, Megan Ruth and members of the Boston Nutrition Obesity Research Center (BNORC; P30 DK046200) for discussion. This work was supported in part by grants from the National Institutes of Health (NCI and NIDDK; R56 DK090455 –GVD) and a subcontract from the Boston Area Diabetes Endocrinology Research Center (BADERC; P30 DK057521). GVD is Chair-Elect of the Obesity and Cancer Section of The Obesity Society. The authors acknowledge the intellectual support of The Obesity Society, which has demonstrated commitment to understand and address the problems and opportunities of bariatric surgery and adipose tissue imaging. The views expressed here do not necessarily reflect those of the NIH or any other organization or funding agency with which the authors are affiliated. The organizations and funding agencies did not play any role in the formulation of the opinions expressed herein, nor share any responsibility for the opinions. Any errors or omissions are of course solely the responsibility of the authors.
References and recommended reading
Papers of particular interest have been highlighted as:
• Of special interest
•• Of outstanding interest
- 1.Finucane MM, Stevens GA, Cowan MJ, et al. Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Body Mass Index). National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9. 1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nikolajczyk BS, Jagannathan-Bogdan M, Denis GV. The outliers become a stampede as immunometabolism reaches a tipping point. Immunol Rev. 2012;249:253–275. doi: 10.1111/j.1600-065X.2012.01142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3•.DeFuria J, Belkina AC, Jagannathan-Bogdan M, et al. B cells regulate the pro-inflammatory T cell balance in obesity and metabolic disease. Proc Natl Acad Sci USA. 2013;110:5133–5138. doi: 10.1073/pnas.1215840110. New evidence for critical roles of immune cell subtypes in metabolic dysfunction in obesity in both humans and rodent models; B cell and T cell functions contribute significantly to metabolic health and immunomodulatory drugs will have value to treat metabolic disease in obesity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 5.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer. 2004;48:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 6.Calle EE, Thun MJ. Obesity and cancer. Oncogene. 2004;23:6365–6378. doi: 10.1038/sj.onc.1207751. [DOI] [PubMed] [Google Scholar]
- 7.Denis GV. Bromodomain coactivators in cancer, obesity, type 2 diabetes and inflammation. Discov Med. 2010;10:489–499. [PMC free article] [PubMed] [Google Scholar]
- 8•.Denis GV, Bowen DJ. Uncoupling obesity from cancer: Bromodomain co-regulators that control networks of inflammatory genes. In: Dannenberg AJ, Berger NA, editors. Energy Balance and Cancer. Vol. 8. Springer; New York: 2013. pp. 61–81. Obesity, Inflammation and Cancer. Provocative discussion of how some environments are obesogenic/diabetogenic and some are carcinogenic for humans. Interestingly, many environments share both characteristics; pathogenesis may be linked through inflammatory mechanisms. [Google Scholar]
- 9.Colditz GA, Wolin KY, Gehlert S. Applying what we know to accelerate cancer prevention. Sci Transl Med. 2012;4:127rv4. doi: 10.1126/scitranslmed.3003218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magro DO, Geloneze B, Delfini R, Pareja BC, Callejas F, Pareja JC. Long-term weight regain after gastric bypass: a 5-year prospective study. Obes Surg. 2008;18:648–651. doi: 10.1007/s11695-007-9265-1. [DOI] [PubMed] [Google Scholar]
- 11.Christou NV, Look D, MacLean LD. Weight gain after short- and long-limb gastric bypass in patients followed for longer than 10 years. Ann Surg. 2006;244:734–740. doi: 10.1097/01.sla.0000217592.04061.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santry HP, Gillen DL, Lauderdale DS. Trends in bariatric surgical procedures. JAMA. 2005;294:1909–1917. doi: 10.1001/jama.294.15.1909. [DOI] [PubMed] [Google Scholar]
- 13.Lubrano C, Mariani S, Badiali M, et al. Metabolic or bariatric surgery? Long-term effects of malabsorptive vs restrictive bariatric techniques on body composition and cardiometabolic risk factors. Int J Obes (Lond) 2010;34:1404–1414. doi: 10.1038/ijo.2010.54. [DOI] [PubMed] [Google Scholar]
- 14•.Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366(17):1577–1585. doi: 10.1056/NEJMoa1200111. Important evaluation of the relative merits of bariatric approaches. [DOI] [PubMed] [Google Scholar]
- 15.Wang F, Deeney JT, Denis GV. Brd2 gene disruption causes ‘metabolically healthy’ obesity: Epigenetic and chromatin-based mechanisms that uncouple obesity from Type 2 diabetes. In: Litwack G, editor. Vit Horm. Vol. 91. Elsevier Academic Press; Amsterdam: 2013. pp. 49–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16••.Bouchard C, Blair SN, Church TS, et al. Adverse metabolic response to regular exercise: is it a rare or common occurrence? PLoS One. 2012;7(5):e37887. doi: 10.1371/journal.pone.0037887. Important and surprising result showing adverse responses to a healthy intervention in an unselected population, in this case, regular exercise. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grundy SM, Cleeman JI, Daniels SR, et al. American Heart Association, diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. Erratum in: Circulation 112, e297–e298. [DOI] [PubMed] [Google Scholar]
- 18.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bastard JP, Maachi M, Lagathu C, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17:4–12. [PubMed] [Google Scholar]
- 20.Klöting N, Fasshauer M, Dietrich A, et al. Insulin-sensitive obesity. Am J Physiol Endocrinol Metab. 2010;299:E506–515. doi: 10.1152/ajpendo.00586.2009. [DOI] [PubMed] [Google Scholar]
- 21.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 22.Wildman RP, Muntner P, Reynolds K, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004) Arch Intern Med. 2008;168:1617–1624. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 23.Sims EA. Are there persons who are obese, but metabolically healthy? Metabolism. 2001;50:1499–1404. doi: 10.1053/meta.2001.27213. Erratum in: Metabolism 2002; 51:536. [DOI] [PubMed] [Google Scholar]
- 24.Blüher M. The distinction of metabolically ‘healthy’ from ‘unhealthy’ obese individuals. Curr Opin Lipidol. 2010;21:38–43. doi: 10.1097/MOL.0b013e3283346ccc. [DOI] [PubMed] [Google Scholar]
- 25.Ruderman NB, Schneider SH, Berchtold P. The metabolically obese, normal-weight individual. Am J Clin Nutr. 1981;34:1617–1621. doi: 10.1093/ajcn/34.8.1617. [DOI] [PubMed] [Google Scholar]
- 26.Ruderman NB, Chisholm D, Pi-Sunyer X, Schneider S. The metabolically obese, normal-weight individual revisited. Diabetes. 1998;47:699–713. doi: 10.2337/diabetes.47.5.699. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka S, Togashi K, Rankinen T, et al. Is adiposity at normal body weight relevant for cardiovascular disease risk? Int J Obes Relat Metab Disord. 2002;26:176–183. doi: 10.1038/sj.ijo.0801880. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka S, Togashi K, Rankinen T, et al. Sex differences in the relationships of abdominal fat to cardiovascular disease risk among normal-weight white subjects. Int J Obes Relat Metab Disord. 2004;28:320–323. doi: 10.1038/sj.ijo.0802545. [DOI] [PubMed] [Google Scholar]
- 29•.Bradshaw PT, Monda KL, Stevens J. Metabolic syndrome in healthy obese, overweight, and normal weight individuals: the Atherosclerosis Risk in Communities Study. Obesity (Silver Spring) 2013;21:203–209. doi: 10.1002/oby.20248. Investigation of the role of lifestyle factors in the stability of the MHO phenotype in racially diverse populations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller MA, Croft LB, Belanger AR, et al. Prevalence of metabolic syndrome in retired National Football League players. Am J Cardiol. 2008;101:1281–1284. doi: 10.1016/j.amjcard.2007.12.029. [DOI] [PubMed] [Google Scholar]
- 31.Matsuzawa Y. Pathophysiology and molecular mechanisms of visceral fat syndrome: the Japanese experience. Diabetes Metab Rev. 1997;13:3–13. doi: 10.1002/(sici)1099-0895(199703)13:1<3::aid-dmr178>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 32.Karelis AD, Messier V, Brochu M, Rabasa-Lhoret R. Metabolically healthy but obese women: effect of an energy-restricted diet. Diabetologia. 2008;51:1752–1754. doi: 10.1007/s00125-008-1038-4. [DOI] [PubMed] [Google Scholar]
- 33.Janiszewski PM, Ross R. Effects of weight loss among metabolically healthy obese men and women. Diabetes Care. 2010;33:1957–1959. doi: 10.2337/dc10-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Unger RH. Minireview: weapons of lean body mass destruction: the role of ectopic lipids in the metabolic syndrome. Endocrinology. 2003;144:5159–5165. doi: 10.1210/en.2003-0870. [DOI] [PubMed] [Google Scholar]
- 35.Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology. 1990;12:1106–1110. doi: 10.1002/hep.1840120505. [DOI] [PubMed] [Google Scholar]
- 36.Silverman JF, O’Brien KF, Long S, et al. Liver pathology in morbidly obese patients with and without diabetes. Am J Gastroenterol. 1990;85:1349–1355. [PubMed] [Google Scholar]
- 37.Nomura H, Kashiwagi S, Hayahi J, et al. Prevalence of fatty liver in a general population of Okinawa, Japan. Jpn J Med. 1988;27:142–149. doi: 10.2169/internalmedicine1962.27.142. [DOI] [PubMed] [Google Scholar]
- 38.Luyckx FH, Desaive C, Thiry A, et al. Liver abnormalities in severely obese subjects: effect of drastic weight loss after gastroplasty. Int J Obes Relat Metab Disord. 1998;22:222–226. doi: 10.1038/sj.ijo.0800571. [DOI] [PubMed] [Google Scholar]
- 39.Bellentani S, Saccoccio G, Masutti F, et al. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med. 2000;132:112–117. doi: 10.7326/0003-4819-132-2-200001180-00004. [DOI] [PubMed] [Google Scholar]
- 40.Iacobellis G. Epicardial and pericardial fat: close, but very different. Obesity (Silver Spring) 2009;17:625–627. doi: 10.1038/oby.2008.575. [DOI] [PubMed] [Google Scholar]
- 41.Iacobellis G, Pistilli D, Gucciardo M, et al. Adiponectin expression in human epicardial adipose tissue in vivo is lower in patients with coronary artery disease. Cytokine. 2005;29:251–255. doi: 10.1016/j.cyto.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 42.Baker AR, Silva NFD, Quinn DW, et al. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc Diabetol. 2006;5:1. doi: 10.1186/1475-2840-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marchington JM, Mattacks CA, Pond CM. Adipose tissue in the mammalian heart and pericardium: structure, foetal development and biochemical properties. Comp Biochem Physiol B. 1989;94:225–232. doi: 10.1016/0305-0491(89)90337-4. [DOI] [PubMed] [Google Scholar]
- 44.Marchington JM, Pond CM. Site-specific properties of pericardial and epicardial adipose tissue: the effects of insulin and high-fat feeding on lipogenesis and the incorporation of fatty acids in vitro. Int J Obes Relat Metab Disord. 1990;14:1013–1022. [PubMed] [Google Scholar]
- 45.Iacobellis G, Corradi D, Sharma AM. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat Clin Pract Cardiovasc Med. 2005;2:536–543. doi: 10.1038/ncpcardio0319. [DOI] [PubMed] [Google Scholar]
- 46.Krumholz HM, Larson M, Levy D. Prognosis of left ventricular geometric patterns in the Framingham Heart Study. J Am Coll Cardiol. 1995;25:879–884. doi: 10.1016/0735-1097(94)00473-4. [DOI] [PubMed] [Google Scholar]
- 47.Iacobellis G, Ribaudo MC, Zappaterreno A, Iannucci CV, Leonetti F. Relation between epicardial adipose tissue and left ventricular mass. Am J Cardiol. 2004;94:1084–1087. doi: 10.1016/j.amjcard.2004.06.075. [DOI] [PubMed] [Google Scholar]
- 48.Nakajima T, Fujioka S, Tokunaga K, Matsuzawa Y, Tarui S. Correlation of intraabdominal fat accumulation and left ventricular performance in obesity. Am J Cardiol. 1989;64:369–373. doi: 10.1016/0002-9149(89)90537-7. [DOI] [PubMed] [Google Scholar]
- 49.Ruberg FL, Chen Z, Hua N, et al. The relationship of ectopic lipid accumulation to cardiac and vascular function in obesity and metabolic syndrome. Obesity (Silver Spring) 2010;18:1116–1121. doi: 10.1038/oby.2009.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aronne LJ, Segal KR. Adiposity and fat distribution outcome measures: assessment and clinical implications. Obes Res. 2002;10 (Suppl 1):14S–21S. doi: 10.1038/oby.2002.184. [DOI] [PubMed] [Google Scholar]
- 51.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 52.Rosito GA, Massaro JM, Hoffmann U, et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117:605–613. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- 53.Iacobellis G, Leonetti F, Singh N, Sharma AM. Relationship of epicardial adipose tissue with atrial dimensions and diastolic function in morbidly obese subjects. Int J Cardiol. 2007;115:272–273. doi: 10.1016/j.ijcard.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 54.Fox CS, Gona P, Hoffmann U, et al. Pericardial fat, intrathoracic fat, and measures of left ventricular structure and function: the Framingham Heart Study. Circulation. 2009;119:1586–1591. doi: 10.1161/CIRCULATIONAHA.108.828970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55•.Lopez-Miranda J, Perez-Martinez P. It is time to define metabolically obese but normal-weight (MONW) individuals. Clin Endocrinol (Oxf) 2013 Feb 27; doi: 10.1111/cen.12181. E-pub ahead of print. Not all lean individuals exhibit healthy metabolism; the evidence discussed in the present review suggests that increased chronic inflammation is a critical contributor to unhealthy metabolism and increased cardiovascular risk in ‘metabolically unhealthy lean’ individuals. [DOI] [PubMed] [Google Scholar]
- 56•.Gómez-Ambrosi J, Silva C, Galofré JC, et al. Body mass index classification misses subjects with increased cardiometabolic risk factors related to elevated adiposity. Int J Obes (Lond) 2012;36:286–294. doi: 10.1038/ijo.2011.100. Subjects classified as lean by BMI criteria, but with increased body fat, show elevated cardiometabolic risk factors; the specific ectopic adipose tissue depots associated with these risks should be evaluated with high resolution imaging techniques, such as MRI. [DOI] [PubMed] [Google Scholar]
- 57.Taguchi R, Takasu J, Itani Y, et al. Pericardial fat accumulation in men as a risk factor for coronary artery disease. Atherosclerosis. 2001;157:203–209. doi: 10.1016/s0021-9150(00)00709-7. [DOI] [PubMed] [Google Scholar]
- 58.Mazurek T, Zhang L, Zalewski A, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]


