Abstract
Background: This study assessed the feasibility of a portable bihormonal closed-loop system at home.
Subjects and Methods: Sixteen pump-treated patients with type 1 diabetes received 48 h of closed-loop therapy with a telemonitored insulin- and glucagon-delivering closed-loop system and 48 h of patient-managed open-loop therapy.
Results: Owing to technical problems in five cases, only 11 patients could be analyzed. Whereas median (interquartile range) glucose levels were not significantly different during Day 1 of open-loop control (OL1) from closed-loop control (CL1) (8.27 [0.83] mmol/L vs. 8.84 [1.47] mmol/L; P=0.206), they were significantly lower during Day 2 of closed-loop control (CL2) versus open-loop control (OL2) (7.70 [2.29] mmol/L vs. 8.84 [0.87] mmol/L; P=0.027). Time spent in euglycemia (3.9–10 mmol/L) was comparable with 67.2% (38.5%) in OL1 versus 79.2% (16.9%) in CL1 (P=0.189) and 66.0% (29.8%) in OL2 versus 76.5% (23.9%) in CL2 (P=0.162). Time spent in hypoglycemia (<3.9 mmol/L) was comparable on Day 1 of control (OL1, 0.68% [8.68%]; CL1, 2.08% [7.61%]; P=0.593) but significantly higher during Day 2 of control (OL2, 0.00% [11.07%]; CL2, 2.8% [9.8%]; P=0.0172) (P=0.017).
Conclusions: Bihormonal closed-loop control is feasible at home, with comparable time in euglycemia to open-loop control and significantly lower median glucose levels on Day 2 of control at the expense of more time in hypoglycemia, albeit still at a very low percentage of time.
Introduction
To date, most closed-loop (CL) experiments have been performed in the clinical research center (CRC) with a duration ranging from overnight control to 51 h of continuous CL control.1–4 CL approaches, which used automated subcutaneous administration of insulin driven by a predictive algorithm, showed increased time spent in target and less time spent in hypoglycemia or decreased frequency of hypoglycemic periods.5,6 However, the algorithms in both of these systems did not automatically determine the mealtime insulin bolus and thus did not fully close the loop. Currently, first attempts at taking CL beyond the confines of the CRC have been published.7
Good glycemic control was achieved in patients with type 1 diabetes using a bihormonal reactive CL system in an experiment of 2 days including six high-carbohydrate meals and exercise.8 In this study, a partial meal-priming bolus of insulin was given manually before each meal. Another CL prototype with a fading memory proportional derivative algorithm resulted in a lower frequency of hypoglycemia compared with CL without subcutaneous glucagon delivery.9 Each meal was announced to the algorithm, and 75% of the usual mealtime insulin bolus was given at the start of the meal. Recently, the results of a dual-hormone delivery CL system were published.10 Glucose control with this CL system was feasible during exercise, dinner, and nighttime. This study was performed in the CRC, and both glucose readings and hormone delivery doses were entered manually.10
We previously reported on a pilot study testing the feasibility of a bihormonal reactive CL prototype without mealtime announcement and fully automated mealtime insulin coverage, demonstrating no difference in venous glucose concentrations in open-loop (OL) control versus CL control after breakfast in six subjects.11 After the algorithm was optimized, the CL system was studied in a setting that included two meal challenges and exercise. Overall, CL glucose control was comparable to OL control.12 The algorithm of this CL system has been improved, and the hardware has been made suitable for ambulant wear by patients. The aim of the current trial was to test this latest-generation CL system for 48 h outside of the CRC at the home of the patient, under free-living conditions.
Subjects and Methods
Participants
Sixteen participants 18–75 years of age with type 1 diabetes, treated with continuous subcutaneous insulin infusion for more than 6 months, having provided written informed consent, participated in this trial, which was approved by the ethics committee of the Academic Medical Center at the University of Amsterdam (Amsterdam, The Netherlands) and was performed in concordance with the Declaration of Helsinki. Participants with impaired hypoglycemia awareness, according to the questionnaire of either Gold et al.13 or Clarke et al.,14 were excluded. Other exclusion criteria were a body mass index above 35 kg/m2, glycosylated hemoglobin level above 11% (110 mmol/mol), pregnancy or breast feeding, and use of corticosteroids. The analysis could only be performed in 11 participants owing to technical failure of the prototype CL system during the trial, unrelated to the functioning of the algorithm. These failures included dysfunction of insulin pumps due to external cable failure in three participants, simultaneous pump and sensor failure in one patient, and a persistent software bug in the system in the first participant. These technical failures resulted in hyperglycemia with a maximal of 20 mmol/L in four of the five patients without signs of ketoacidosis and hypoglycemia of 2.8 mmol/L that was easily resolved by oral carbohydrates in one patient.
Study design
A single-center, interventional, nonrandomized trial was conducted to compare OL versus bihormonal reactive CL control without mealtime or exercise announcement during a 60-h intervention period. The period between the OL and the CL was more than 3 weeks.
OL control
All patients started with the OL control experiment. A continuous glucose monitor (CGM) (CGMS® System Gold™; Medtronic MiniMed, Sylmar, CA) was inserted, and participants were instructed to calibrate the device according to the manufacturer's specifications. The participants then went home. During OL control, participants were blinded for the results of the CGM and administered their insulin as usual.
CL control
The CL phase consisted of glucose control during 60 h. The first 12 h of the CL intervention took place at the CRC in order to optimize the individually determined algorithm parameter settings, and the last 48-h period of CL control was conducted at the patient's home under free-living conditions. Patients were allowed to leave their houses. Algorithm parameters were initially set based on the patient's total daily insulin need as marker for insulin sensitivity. If postprandial glucose levels increased more than 5 mmol/L, the insulin sensitivity factor was decreased. If the increase was less than 3.5 mmol/L consistently over three meals, the insulin sensitivity factor was increased. If needed, the initial settings could be further changed after 24 h. This was done manually and occurred in two patients.
Patients performed self-measurement of blood glucose (SMBG) before each meal, 2 h postprandially, before leaving the CRC or home, before exercise, before bedtime, at 3 a.m., and in the case of patient-perceived hypo- or hyperglycemia.
During CL control, the patients wore two D-Tron+ pumps (Disetronic Medical Systems, St. Paul, MN) for automated subcutaneous insulin (NovoRapid®; Novo Nordisk, Bagsvaerd, Denmark) and glucagon (Glucagen®; Novo Nordisk) administration and two Medtronic CGM sensors: one primary and one back-up, in case of failure of the first sensor. Four patients completed the trial using the Medtronic (Minneapolis, MN) SofSensor®, and six patients used the Medtronic Enlite® sensor. The Medtronic CGM sensors and the D-Tron+ pumps were connected using partly proprietary interface technologies to a personal computer loaded with the Windows 7 Professional Operating System (Microsoft Inc., Redmond, WA), on which the algorithm software ran. No mealtime or exercise announcements were made to the algorithm.
Algorithm
The algorithm was designed and patented by Inreda Diabetic B.V., Goor, The Netherlands (patent number NL 1032756 in The Netherlands and patent number WO 2007/049961 A3 worldwide). It has been described before.11,12 In brief, the algorithm can be characterized as a individualized, proportional derivative controller. The insulin and glucagon delivery was fully automated and based on the continuous glucose reading of the sensor. There was no basal insulin rate. Insulin delivery was determined by the difference between current and target glucose levels, glucose rate of change, insulin sensitivity of the patient, and two glucose thresholds triggering the delivery of a corrective insulin bolus.
The proportional derivative aspect of the algorithm is determined by the difference between current and target glucose levels and the rate of change of glucose levels according to the formula mL (units) per rise in mmol/L per unit of time (a third-degree polynomial, to be programmed by means of three calibration points+the zero point=target value). The third-degree polynomial thereby acts like an integral component.
Furthermore, insulin delivery was adapted to individually determined insulin sensitivity, and an insulin correction bolus was injected whenever CGM glucose values exceeded 6.5 mmol/L. An additional insulin correction bolus was given if the glucose values exceeded 13.0 mmol/L. If CGM values were falling, the administration of insulin was stopped according to the formula mL (units) per fall in mmol/L per unit of time.
Glucagon administration was started if the CGM glucose level fell below 6.5 mmol/L. A bolus of glucagon was injected depending on the rate of fall of glucose value, followed by glucagon delivery according to an exponential glucagon injection curve. The amounts of glucagon were small relative to the typical 1-mg dose used to treat severe hypoglycemia and were derived from glucagon administration in healthy volunteers.15 Below a glucose concentrations of 4.0 mmol/L, a larger bolus of glucagon was administered. The concentration of glucagon was 1 mg/mL. As a precaution, no more than 1 mg of glucagon/h could be administered. Because of the instability of glucagon, it was reconstituted for use every 24 h.
The system was idle when the rate of change was less than 2 mmol/L/h. Frequency of adjustments in insulin or glucagon administration was based on the rate of change of CGM glucose level. Control intervals, that is, the frequency of a controller decision to deliver insulin or glucagon, ranged from once every 6 min to once every 15 min, being more often when the rate of change of glucose level increased.
In case of hypoglycemia <3.5 mmol/L, an auditory alarm was activated advising the patient to take oral carbohydrates. All hypoglycemic alarms were confirmed with SMBG. If SMBG values were between 3 and 4 mmol/L, 12 g of carbohydrates was taken, and if values were below 3.0 mmol/L, 18 g of carbohydrates was taken.
Safety
To maximize patient safety, the complete CL system was designed with separately operating subsystems: one subsystem contained the glycemic controller, and the other subsystem contained the safety processor for the two pumps and batteries. Furthermore, the CL system used both a primary sensor and a back-up sensor. As an additional precaution during the CL experiments, CGM output was monitored remotely 24 h/day via a Wi-Fi and 3G cellular-based telemedicine system. To further reduce the risk of hypo- or hyperglycemia, several alerts were built into the system:
• High glucose alert: the system gave an alert in case the glucose level exceeded 15 mmol/L.
• Occlusion alert: the system gave an alert in case the insulin or glucagon pump occluded.
• Insulin/glucagon administration alert: the system gave an alert if more than a predetermined amount of insulin or glucagon was infused over an hour and also calculated over a 24-h period to prevent insulin overdosing and minimize hyper- or hypoglycemia.
• Eating alert: the system advised oral carbohydrate intake in case the glucose level went below 3.5 mmol/L.
• Sensor alert: an alert was given if the difference between the two sensors was more than 20% below 8 mmol/L and in case of a difference of more than 1.5 mmol/L above 8 mmol/L.
Patients refrained from operating a motorized vehicle during the duration of the trial. A dedicated mobile phone was provided to enable direct communication between each participant and the research team.
CGM calibration procedure
Calibration was performed before starting CL control and was repeated in case of a SMBG to sensor difference exceeding 1.5 mmol/L. The calibration procedure consisted of taking three concomitant CGM and SMBG values at 5-min intervals. From these, an average conversion factor was derived. The system measured the sensor current directly and converted this into glucose values. In case a new CGM was placed, the CGM was calibrated after a 3-h warm-up period.
Statistics
The first 12-h interval of the CL system was used to set individual parameters and therefore excluded from analysis. The remaining 48-h interval was divided in two 24-h periods for statistical analysis. This was done because after the conclusion of the first 24-h period adjusting the patient's insulin sensitivity parameters was allowed, influencing CL control. Thus, Day 1 of OL control (OL1) was compared with Day 1 of CL control (CL1), and Day 2 of OL control (OL2) was compared with Day 2 of CL control (CL2). The primary outcome was the proportion of time spent in euglycemia (glucose concentration between 3.9 mmol/L and 10 mmol/L). Secondary outcomes included time spent in hypoglycemia (glucose concentration <3.9 mmol/L) and hyperglycemia (glucose concentration >10 mmol/L).
A secondary analysis was performed assessing control during the postprandial periods (3 h after every meal) and the nighttime periods (between 12 a.m. and 7 a.m.). It was originally planned to analyze the post-exercise period, but this could not be analyzed as most patients did not wear the heart rate belt to have a sufficient amount of time to allow for data analyses.
The proportion of time spent in euglycemia, hypoglycemia, and hyperglycemia, amount of insulin, variability measured as SD, and the number of hypoglycemic episodes were compared using the t test for paired measurements or Wilcoxon signed ranks test, depending on the distribution of the data. Outcome measures were analyzed for significance (P<0.05) using SPSS version 20 software (IBM Corp., Armonk, NY) and GraphPad Prism® 5 software (GraphPad Software Inc., San Diego, CA).
Results
Baseline characteristics
Four of the 11 participants were female. Their mean age (range) was 52.1 (25–66) years, and their mean glycosylated hemoglobin level was 7.6% (6.2–9.5%). Mean diabetes duration was 35.3 (12–53) years, and mean duration of continuous subcutaneous insulin infusion use was 11.2 (4–17) years.
Time spent in euglycemia
Median (interquartile range [IQR]) time spent in euglycemia was comparable with 67.2% (38.5%) in OL1 versus 79.2% (16.9%) in CL1 (P=0.1891) and 66.0% (29.8%) in OL2 versus 76.5% (23.9%) in CL2 (P=0.1618).
Time spent in hypoglycemia
Median (IQR) time spent in hypoglycemia was comparable at Day 1 (OL1 vs. CL1, 0.7% [8.7%] vs. 2.1% [7.6%]; P=0.5934) but significantly higher with CL use on Day 2 (OL2 vs. CL2, 0.0% [11.1%] vs. 2.8% [9.8%]; P=0.0172).
Time spent in hyperglycemia
Time spent in hyperglycemia was comparable on all days: OL1 versus CL1, 32.4% (44.8%) versus 13.2% (16.1%) (P=0.1678); and OL2 versus CL2, 31.0% (29.8%) versus 18.3% (20.0%) (P=0.0889).
Median glucose levels
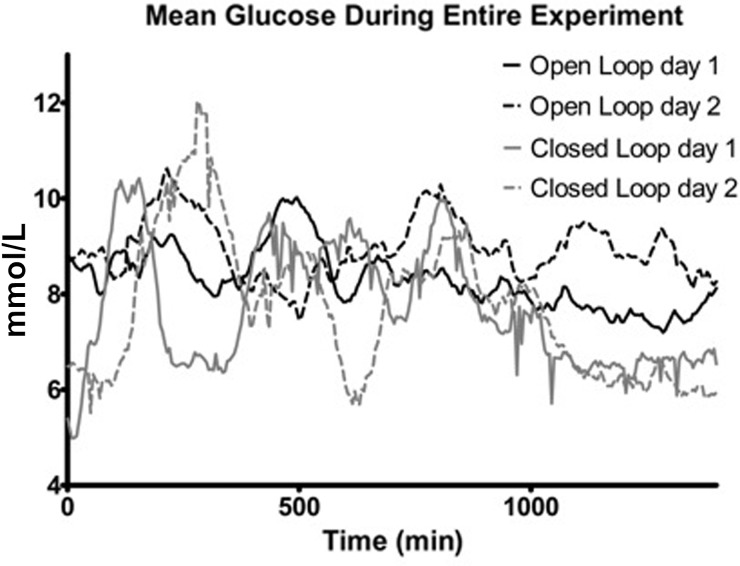
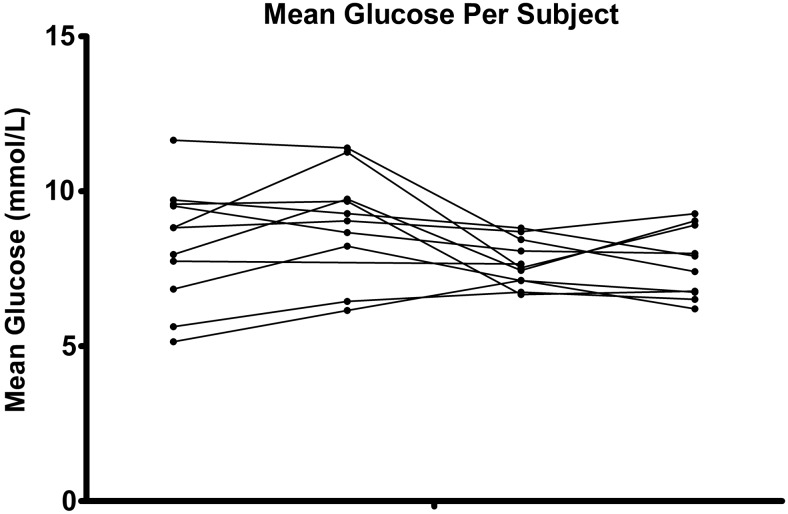
Median glucose levels were comparable on Day 1 (OL1 vs. CL1, 8.27 [0.83] mmol/L vs. 7.38 [2.23] mmol/L; P=0.2061) but significantly lower with use of CL on Day 2 (OL2 vs. CL2, 8.84 [0.87] mmol/L vs. 7.70 [2.29] mmol/L; P=0.0273) (Fig. 1). Except for the nighttime on Day 2, median glucose levels of all other periods were comparable (Table 1). Individual data are represented as a period-to-period line curve in Figure 2. Glucose variability was measured by SD and is given in Table 2. No differences were seen between OL and CL.
FIG. 1.
Mean glucose levels during the first and last 24 h of open-loop and closed-loop control.
Table 1.
Median (Interquartile Range) Glucose Levels for the First and Last 24 H of Open-Loop and Closed-Loop Control
| Glucose levels (mmol/L) [median (IQR)] | ||||||
|---|---|---|---|---|---|---|
| OL1 | CL1 | P | OL2 | CL2 | P | |
| Entire experiment | 8.27 (0.83) | 7.38 (2.23) | 0.2061 | 8.84 (0.87) | 7.70 (2.29) | 0.0273 |
| Night only | 7.68 (0.44) | 6.59 (0.20) | 0.2783 | 8.78 (0.71) | 6.17 (0.38) | 0.0039 |
| Breakfast | 8.87 (1.97) | 8.90 (2.59) | 1.0 | 10.29 (1.43) | 9.8 (3.18) | 0.6523 |
| Lunch | 9.48 (1.73) | 9.07 (2.02) | 0.5566 | 8.83 (0.44) | 10.49 (3.62) | 0.8203 |
| Dinner | 8.99 (1.00) | 8.46 (1.39) | 0.7695 | 9.76 (0.86) | 8.56 (0.88) | 0.7344 |
CL1, first closed-loop 24-h interval; CL2, last closed-loop 24-h interval; IQR, interquartile range; OL1, first open-loop 24-h interval; OL2, last open-loop 24-h interval.
FIG. 2.
Individual curves per period-to-period line curve. The sequence for the black dots is: open-loop, Day 1; open-loop, Day 2; closed-loop, Day 1; and closed-loop, Day 2.
Table 2.
Glucose Variability Measured as SD
| Mean | Minimum | Maximum | P value | |
|---|---|---|---|---|
| OL1 | 2.65 | 0.81 | 3.91 | |
| CL1 | 2.63 | 1.51 | 3.97 | 0.859 |
| OL2 | 2.39 | 0.82 | 3.67 | |
| CL2 | 2.99 | 1.69 | 5.36 | 0.203 |
CL1, first closed-loop 24-h interval; CL2, last closed-loop 24-h interval; OL1, first open-loop 24-h interval; OL2, last open-loop 24-h interval.
Outcome nighttime values
During the nighttime, the median glucose levels were significantly lower on Day 2 of CL use in comparison with Day 2 of OL control: OL2 versus CL2, 8.78 (0.71) mmol/L versus 6.17 (0.38) mmol/L (P=0.0039).
Median (IQR) time spent in euglycemia was significantly different: 69% (34.6%) in OL1 versus 97.1% (8.3%) in CL1 (P=0.03) and 88.4% (43.1%) in OL2 versus 100% (12.3%) in CL2 (P=0.23).
Median (IQR) time spent in hypoglycemia was comparable on Night 1 (OL1 vs. CL1, 0% [24.9%] vs. 0% [8.1%]; P=0.59) and on Night 2 (OL2 vs. CL2, 0.0% [0%] vs. 0% [4.1%]; P=not applicable). Time spent in hyperglycemia was comparable in both nights: OL1 versus CL1, 21.4% (35%) versus 2.9% (4.8%) (P=0.3); and OL2 versus CL2, 11.6% (43.1%) versus 0% (12.2%) (P=0.2).
Safety data
No differences were seen in oral carbohydrate administration between OL and CL control. In OL use, oral carbohydrates were administered six times in four persons during the first 24 h and six times in six persons during the last 24 h. In CL use, this occurred eight and nine times in five and seven persons, respectively (P=0.76 and P=0.32, respectively).
Sensor and algorithm performance
The mean absolute relative difference of the sensor is given in Table 3. No differences were seen between OL and CL control. Extreme errors are given in Table 4.
Table 3.
Mean Absolute Relative Difference of Continuous Glucose Monitoring During the Whole Experiment
| Mean | Minimum | Maximum | P value | |
|---|---|---|---|---|
| OL1 | 0.158 | 0.03 | 0.377 | |
| CL1 | 0.198 | 0.09 | 0.299 | 0.307 |
| OL2 | 0.180 | 0.068 | 0.423 | |
| CL2 | 0.207 | 0.1 | 0.593 | 1.0 |
Mean absolute relative difference is defined as (sensor glucose – self-monitored blood glucose)/sensor glucose.
CL1, first closed-loop 24-h interval; CL2, last closed-loop 24-h interval; OL1, first open-loop 24-h interval; OL2, last open-loop 24-h interval.
Table 4.
Frequency of Extreme Errors of Continuous Glucose Monitoring Defined as Mean Number of Paired Samples During the Whole Experiment
| Extreme error | OL1 | OL2 | CL1 | CL2 |
|---|---|---|---|---|
| >30% | 2 | 1 | 1 | 0 |
| >50% | 0 | 0 | 0 | 1 |
CL1, first closed-loop 24-h interval; CL2, last closed-loop 24-h interval; OL1, first open-loop 24-h interval; OL2, last open-loop 24-h interval.
The CGM was recalibrated a total of 24 times during CL because of difference in glucose values exceeding 1.5 mmol/L between the two sensors or between SMBG and CGM, and in 16 times the back-up sensor was needed. Four times replacement of the CGM was necessary owing to technical failure.
Three times an occlusion alert was given, and once the insulin administration alert was given. All insulin or glucagon calls were administered. Twelve times during CL control, the high glucose alert went off. The wireless connection was lost a total of seven times. In one instance the cables were disconnected, and in one instance the batteries were empty. All these problems could be resolved quickly with no deleterious effects to study subjects. Beside these problems, no systems interventions were necessary.
Infused amount of insulin
The infused amount of insulin was significantly higher on Day 1 of use of the CL system (OL1 vs. CL1, 34.7 [22.8] IU vs. 52.0 [29.0] IU; P=0.001) but comparable on Day 2 (OL2 vs. CL2, 37.6 [31.7] IU vs. 50.7 [39.9] IU; P=0.1055).
No differences were seen in amount of administered insulin between OL and CL use for both days: on Day 1 of use of the CL system, OL1 versus CL1, 6 (3.0) IU versus 6.3 (3.3) IU (P=0.57); and on Day 2, OL2 versus CL2, 6.6 (3.7) IU versus 6.6 (3.4) IU (P=0.86).
Infused glucagon
The infused amount of glucagon was comparable on both the CL days (CL1 vs. CL2, 2.7 (2.0) mg vs. 1.7 [1.5] mg). The dose of glucagon during the night on CL1 (0.6 [0.4] mg) and CL2 (0.6 [0.5] mg) was identical (P=0.86).
No side effects were seen. In total, 0.3 glucagon instances per hour were given, of which 0.05 glucagon instances per hour were followed by 17 hypoglycemia alerts.
Discussion
This small study is the first to show that full-day CL control is achievable at the patient's home under free-living conditions with glycemic results comparable to OL control with respect to median glucose values and time spent in euglycemia. In absolute numbers, significantly more periods of hypoglycemia occurred on Day 2 in CL. It could be argued that the CGM underestimated time in hypoglycemia during Day 2 of OL because all six hypoglycemic episodes noted by the patients on Day 2 of OL were missed by the CGM.
This study was limited by typical technical issues related to the prototype, which reduced the number of sessions available for statistical analysis. Most of these technical issues were caused by damage to cables connecting the platform to the insulin pumps and wireless communication problems. This can be remedied by re-engineering the system with integration of all CL components into a smaller dedicated device with only the sensor communicating wirelessly.
However, aforementioned difficulties do not negate the fact that glycemic control in those patients who were treated in CL with a fully functional system was comparable to OL, although it was a small study of short duration. Therefore longer experiments are needed to assess within-subject variability over subsequent days.
The calibration procedure in OL and CL use was different, and in theory this could have affected the outcome. However, because the CGM mean absolute relative difference was similar in OL and CL control, we do not think this will have materially affected the outcome. Another limitation was the lack of randomization, so a time effect cannot be excluded. Because of logistical reasons, randomization was not possible, but a washout period of at least 3 weeks between OL and CL minimized the likelihood of any carryover effect.
CL control was achieved using a system without any form of premeal insulin bolus, meal, or exercise announcement and that can therefore be classified as a fully CL system. Additionally, during the trial patients were not instructed with regard to their meals or exercise. In other words, they could eat and do as much as they wanted, whenever they wanted. This led to several patients enjoying copious meals served with alcoholic beverages while they were attending social gatherings, with no apparent effect on CL control.
Although we did not formally examine patient satisfaction, all patients expressed they were very content with the system despite its format as large as a briefcase. They felt for a short time relieved of their disease.
Clearly, CL control in the CRC is an entirely different situation than CL control at home. Whereas patients are usually more compliant with diet and exercise advice when participating in a clinical trial (the Hawthorne effect),16 here the opposite seemed to occur, when patients apparently wanted to try out things they knew would be challenging for the CL to deal with.
The transition of CRC-tested CL systems to the patients' homes is one of the major challenges facing CL research today. Systems need to be reliable and error free and be able to cope with daily patient life. Furthermore, we believe that CL systems should not depend on the patient for meal and exercise information. As we work to reduce the size and possibility of technical failure, we can conclude that CL control at home is achievable and safe and results in glycemic control comparable to OL control.
Acknowledgments
Medtronic Europe Sarl provided the Medtronic SofSensors MMT-7002C sensors free of charge. The Medtronic Enlite Sensors were bought through regular channels. Further development of this closed-loop system is supported by grant 305654 from the European Commission to the PCDIAB Consortium.
Author Disclosure Statement
R. Koops and R. Koebrugge are employees of Inreda BV, Goor, The Netherlands. A.C.vanB., Y.M.L., J.B.L.H., and J.H.deV. have no competing financial interests.
A.C.vanB. contributed to literature search, study design, data collection, analysis, and interpretation and wrote the manuscript. Y.M.L. contributed to the literature search, data collection, analysis, and interpretation and wrote the manuscript. R. Koebrugge contributed to the study design and data collection and edited the manuscript. R. Koops contributed to the study design and data collection and edited the manuscript. J.B.L.H. contributed to the study design and reviewed/edited the manuscript. J.H.deV. supervised the study design and research and reviewed/edited the manuscript.
References
- 1.Breton M, Farret A, Bruttomesso D, Anderson S, Magni L, Patek S, Dalla Man C, Place J, Demartini S, Del Favero S, Toffanin C, Hughes-Karvetski C, Dassau E, Zisser H, Doyle FJ, 3rd, De Nicolao G, Avogaro A, Cobelli C, Renard E, Kovatchev B; International Artificial Pancreas Study Group: Fully integrated artificial pancreas in type 1 diabetes: modular closed-loop glucose control maintains near normoglycemia. Diabetes 2012;61:2230–2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clarke WL, Anderson S, Breton M, Patek S, Kashmer L, Kovatchev B: Closed-loop artificial pancreas using subcutaneous glucose sensing and insulin delivery and a model predictive control algorithm: the Virginia experience. J Diabetes Sci Technol 2009;3:1031–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hovorka R: Closed-loop insulin delivery: from bench to clinical practice. Nat Rev Endocrinol 2011;7:385–395 [DOI] [PubMed] [Google Scholar]
- 4.Kovatchev B, Cobelli C, Renard E, Anderson S, Breton M, Patek S, Clarke W, Bruttomesso D, Maran A, Costa S, Avogaro A, Dalla Man C, Facchinetti A, Magni L, De Nicoloa G, Place J, Farret A: Multinational study of subcutaneous model-predictive closed-loop control in type 1 diabetes mellitus: summary of the results. J Diabetes Sci Technol 2010;4:1374–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hovorka R, Allen JM, Elleri D, Chassin LJ, Harris J, Xing D, Kollman C, Hovorka T, Larsen AM, Nodale M, De Palma A, Wilkinska ME, Acerini CL, Dunger DB: Manual closed-loop insulin delivery in children and adolescents with type 1 diabetes: a phase 2 randomised crossover trial. Lancet 2010;375:743–751 [DOI] [PubMed] [Google Scholar]
- 6.Renard E; AP@home consortium: Time in hypoglycemia in patients with type 1 diabetes is dramatically reduced when insulin infusion is driven by two closed-loop algorithms in a randomised clinical trial [abstract]. Diabetologia 2012;55(Suppl 1):S86 [Google Scholar]
- 7.Philip M, Battelino T, Atlas E, Kordonouri O, Bratina N, Miller S, Biester T, Stefanija MA, Muller I, Nimri R, Danne T: Nocturnal glucose control with an artificial pancreas at a diabetes camp. N Engl J Med 2013;368:824–833 [DOI] [PubMed] [Google Scholar]
- 8.Russell SJ, El-Khatib FH, Nathan DM, Magyar KL, Jiang J, Damiano ER: Blood glucose control in type 1 diabetes with a bihormonal bionic endocrine pancreas. Diabetes Care 2012;35:2148–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castle JR, Engle JM, El YJ, Massoud RG, Yuen KC, Kagan R, Ward WK: Novel use of glucagon in a closed-loop system for prevention of hypoglycemia in type 1 diabetes. Diabetes Care 2010;33:1282–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haidar A, Lequalt L, Dallaire M, Alkhateeb A, Coriati A, Messier V, Cheng P, Millette M, Boulet B, Rabasa-Lhoret R: Glucose-responsive insulin and glucagon delivery (dual-hormone artificial pancreas) in adults with type 1 diabetes: a randomized crossover controlled trial. CMAJ 2013;185:297–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Bon AC, Hermanides J, Koops R, Hoekstra JB, Devries JH: Postprandial glycemic excursions with the use of a closed-loop platform in subjects with type 1 diabetes: a pilot study. J Diabetes Sci Technol 2010;4:923–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Bon AC, Jonker LD, Koebrugge R, Koops R, Hoekstra JB, Devries JH: Feasibility of a bihormonal closed-loop system to control postexercise and postprandial glucose excursions. J Diabetes Sci Technol 2012;6:1114–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gold AE, MacLeod KM, Frier BM: Frequency of severe hypoglycemia in patients with type I diabetes with impaired awareness of hypoglycemia. Diabetes Care 1994;17:697–703 [DOI] [PubMed] [Google Scholar]
- 14.Clarke WL, Cox DJ, Gonder-Frederick LA, Julian D, Schlundt D, Polonsky W: Reduced awareness of hypoglycemia in adults with IDDM. A prospective study of hypoglycemic frequency and associated symptoms. Diabetes Care 1995;18:517–522 [DOI] [PubMed] [Google Scholar]
- 15.Graf CJ, Woodworth JR, Seger ME, Holcombe JH, Bowsher RR, Lynch R: Pharmacokinetic and glucodynamic comparisons of recombinant and animal-source glucagon after IV, IM, and SC injection in healthy volunteers. J Pharm Sci 1999;88:991–995 [DOI] [PubMed] [Google Scholar]
- 16.Devries JH, Snoek FJ, Kostense PJ, Heine RJ: Improved glycaemic control in type 1 diabetes patients following participation per se in a clinical trial—mechanisms and implications. Diabetes Metab Res Rev 2003;19:357–362 [DOI] [PubMed] [Google Scholar]