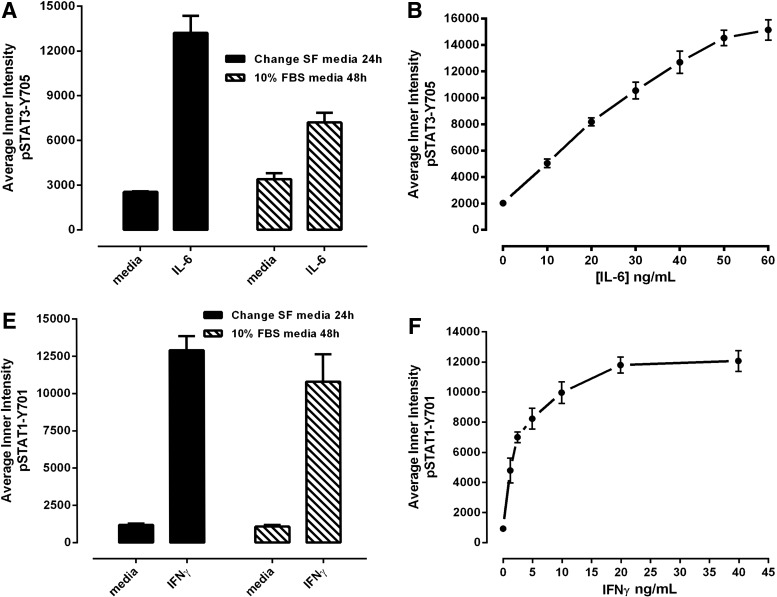
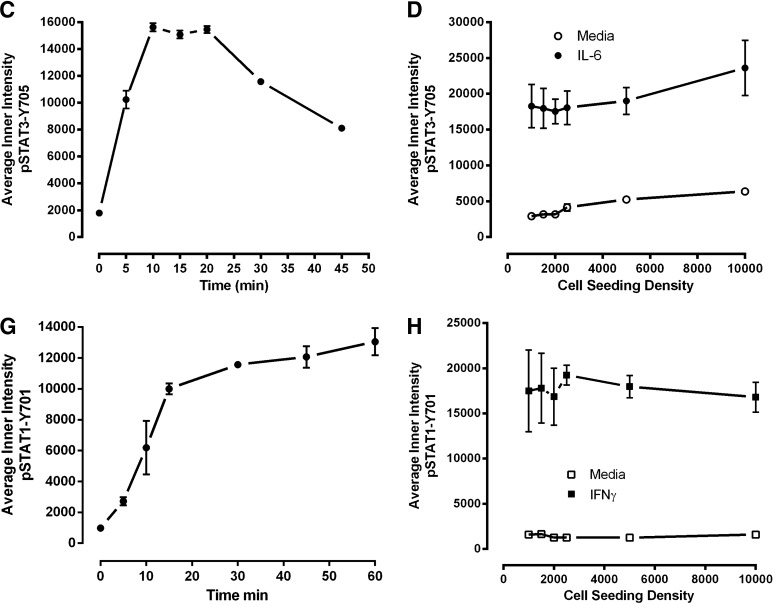
Fig. 3.
Development and optimization of the pSTAT3-Y705 and pSTAT1-Y701 HCS assays. Treated Cal33 HNSSC cells were fixed and stained with Hoechst and either a mouse monoclonal anti-p-STAT3-Y705 (A–D) antibody or a mouse monoclonal anti-p-STAT1-Y701 (E–H) as described in Materials and Methods (Table 1). Images of two fields of view for two fluorescent channels, Hoechst (Ch 1) and either pSTAT3-Y705-FITC or pSTAT1-Y701-FITC (Ch 2), were acquired on the IXU confocal HCS platform using a 20×/0.45NA objective, and the quantitative data presented were extracted from the digital images using the TE image analysis module as described in Materials and Methods. Serum-free media exchange, (A) IL-6 and pSTAT3-Y705 and (E) IFNγ and pSTAT1-Y701 HCS. First, 2,000 Cal33 HNSSC cells were seeded into the wells of duplicate 384-well microtiter plates in DMEM containing 10% FBS and cultured overnight in an incubator at 37°C in 5% CO2 and 95% humidity. In one of the duplicate plates, the cell culture medium was then aspirated, monolayers were washed once with SFM, fresh SFM was added to the wells, and the plates were returned to the incubator at 37°C in 5% CO2 and 95% humidity for an additional 24 h. The other plate was left in the incubator untouched. After 24 h, both the serum-containing and serum-free plates of Cal33 cells were then treated with SFM, 50 ng/mL of IL-6, or 30 ng/mL IFNγ for 15 min at 37°C in 5% CO2 and 95% humidity and were then fixed and stained with Hoechst and either a mouse monoclonal anti-p-STAT3-Y705 antibody or a mouse monoclonal anti-p-STAT1-Y701 antibody as described in Materials and Methods (Table 1). The mean±SD (n=6) average inner (nuclear) intensities of the p-STAT3-Y705 or p-STAT1-Y701 signals from six wells treated with medium or cytokine in cells cultured in serum containing (filled bars) conditions throughout, or serum starved for 24 h (hashed bars) before cytokine treatment are presented. Representative experimental data from one of three independent experiments are shown. Cytokine concentration responses, (B) IL-6 and pSTAT3-Y705 and (F) pSTAT1-Y701 HCS. First, 2,000 Cal33 HNSSC cells that had been serum starved for 24 h as described earlier were treated with the indicated concentrations of IL-6 or IFNγ for 15 min at 37°C in 5% CO2 and 95% humidity, and the treated cells were then fixed and stained with Hoechst and either a mouse monoclonal anti-p-STAT3-Y705 antibody or a mouse monoclonal anti-p-STAT1-Y701 antibody as described in Materials and Methods (Table 1). The mean±SD (n=4) average inner (nuclear) intensities of the p-STAT3-Y705 (B, •) or p-STAT1-Y701 (F, •) signals from four wells treated with each cytokine concentration are presented. Representative experimental data from one of four independent experiments are shown. Cytokine time courses, (C) IL-6 and pSTAT3-Y705 and (G) IFNγ and pSTAT1-Y701 HCS. First, 2,000 Cal33 HNSSC cells that had been serum starved for 24 h as described earlier were treated with 50 ng/mL of IL-6 or 30 ng/mL IFNγ for the indicated time periods at 37°C in 5% CO2 and 95% humidity, and the treated cells were then fixed and stained with Hoechst and either a mouse monoclonal anti-p-STAT3-Y705 antibody or a mouse monoclonal anti-p-STAT1-Y701 antibody as described in Materials and Methods (Table 1). The mean±SD (n=8) average inner (nuclear) intensities of the p-STAT3-Y705 (C, •) or p-STAT1-Y701 (G, •) signals from eight wells treated with each cytokine concentration are presented. Representative experimental data from one of four independent experiments are shown. Cal33 HNSSC cell seeding density, (D) IL-6 and pSTAT3-Y705 and (H) IFNγ and pSTAT1-Y701 HCS. Cal33 HNSSC cells that had been seeded into the wells of 384-well microtiter plates at the indicated cell seeding densities in serum-containing medium for 24 h and then serum starved for 24 h as described earlier were then treated with medium, 50 ng/mL of IL-6, or 30 ng/mL IFNγ for 15 min at 37°C in 5% CO2 and 95% humidity, and were then fixed and stained with Hoechst and either a mouse monoclonal anti-p-STAT3-Y705 antibody or a mouse monoclonal anti-p-STAT1-Y701 antibody as described in Materials and Methods (Table 1). The mean±SD (n=6) average inner (nuclear) intensities of the p-STAT3-Y705 (D, ◯ medium, • IL-6) or p-STAT1-Y701 (H, ◯ medium, ■ IFNγ) signals from six wells treated with each cytokine are presented. Representative experimental data from one of three independent experiments are shown. IFNγ, interferon gamma.