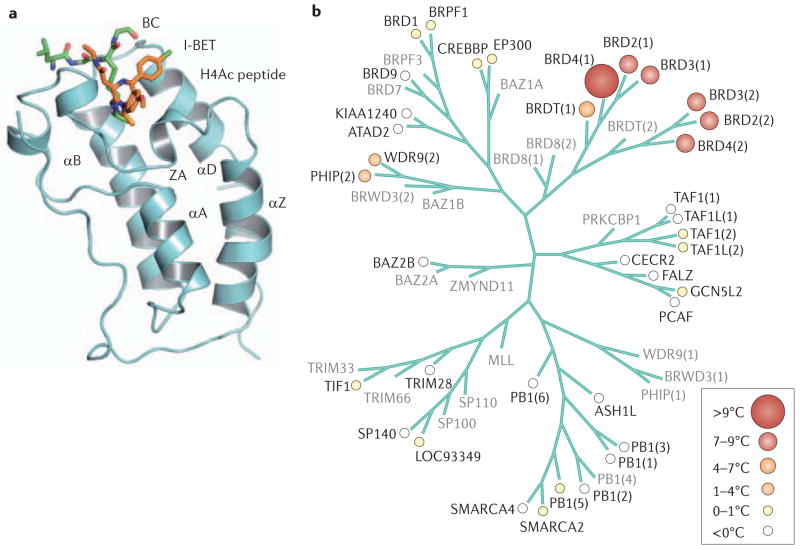
Figure 1. Structure and relationships among bromodomain-containing proteins.
a. The anti-parallel α-helices of the bromodomain bundle are shown in association with the small-molecule inhibitor I-BET and a histone H4 lysine peptide acetylated at position 12 (REF. 97). The BC and ZA loops form the binding pocket for the ε-acetyl-lysine groups of nucleosomal histones in the structure, which the Zhou group first described in detail13. b. Relatedness among bromodomain families, as defined by selectivity for JQ1, is measured by differential scanning fluorimetry79. The BET proteins BRD2, BRD3 and BRD4 are shown to be closely related, with respect to both the first and second bromodomains, as well as the first bromodomain of BRDT. The second bromodomain of BRDT was not tested (shown in grey). Part a is reproduced, with permission, from REF. 97 © (2010) Macmillan Publishers Ltd. All rights reserved. Part b reproduced, with permission, from REF. 79 © (2010) Macmillan Publishers Ltd. All rights reserved.