Fig. 1.
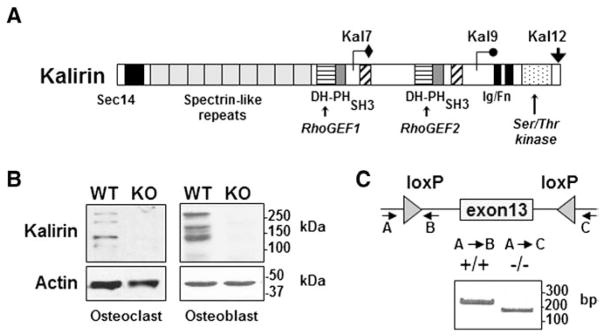
Expression of Kalirin in osteoclasts and osteoblasts. (A) Schematic representation of the three major Kalirin isoforms formed by alternative mRNA splicing. All full-length isoforms contain a Sec14 lipid binding domain, nine spectrin repeats and a Rho-GEF (PH-DH) domain. Kal9 also contains an SH3 domain and the second GEF domain. Kal12 contains two SH3 domains and a second GEF domain as well as an Ig/FnIII domain and Ser/Thr kinase domain. (B) Osteoclasts were differentiated from non-adherent bone marrow cells in the presence of MCSF and RANKL, while osteoblasts were obtained from cultures of neonatal calvaria preparations. Kalirin isoforms were identified by Western blotting using an antibody that detects the spectrin domain which is common to each isoform [32]. Protein lysates were prepared from WT mice and from mice lacking Kalirin (Kal-KO). Actin was used as a protein loading control. Molecular mass markers are indicated. (C) Global Kal-KO mice were generated by crossing Kalrn exon 13 floxed mice with Cre-Hprt mice to excise Exon 13 of the Kalirin gene [31]. PCR analysis of rat tail DNA was used to confirm deletion of Kalirin mRNA in mice. The migration of PCR products is indicated by the DNA ladder (base pairs; bp). Western blotting (B) also confirmed the absence of Kalirin isoforms in Kal-KO osteoclasts and osteoblasts.
