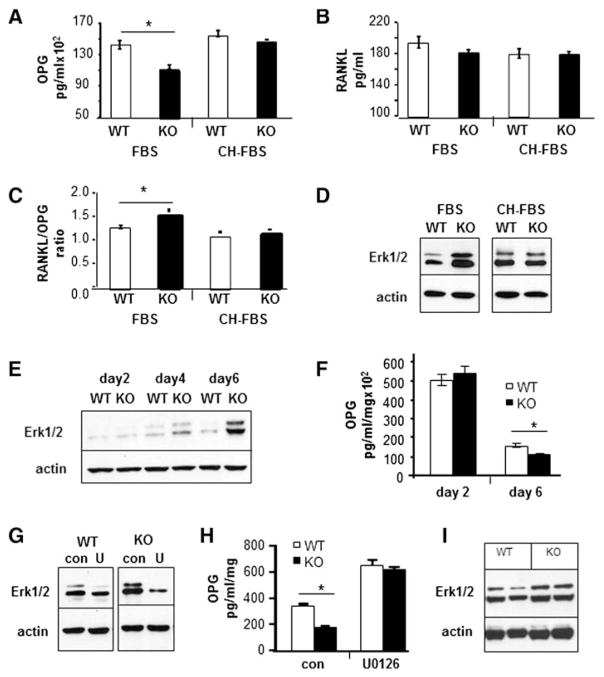
Fig. 8.
Loss of Kalirin affects ERK signaling and OPG secretion. (A–C) Osteoblasts were cultured for 1 week in growth media and then replaced with media containing either 10% FBS or 10% CH-FBS for 24 h. Conditioned media was collected and assayed for OPG or RANKL, and the ratio of RANKL to OPG was determined as indicated. (D) Cells grown in media containing 10% FBS or 10% CH-FBS were lysed and subject to SDS-PAGE and Western blotting with an antibody to phosphorylated ERK1/2. The doublet reflects the p42/p44 isoforms of ERK. Actin was used as a loading control. (E) Osteoblasts from WT and Kal-KO mice were cultured in growth media for 2, 4 or 6 days, followed by Western blot analysis with an antibody to phosphorylated ERK1/2. (F) Media were changed daily and conditioned media collected at day 2 and day 6 were assayed using the OPG immunoassay. (G) Day 6 osteoblast cultures were treated with vehicle or the ERK inhibitor U0126 (10 μM) for 24 h, followed by Western blotting for phosphorylated ERK1/2 or actin. (H) Media were collected after 24 h treatment of osteoblasts with U0126 and assayed for OPG levels. Results were normalized for total protein. Three wells per condition were used and OPG or RANKL was assayed in duplicate. (I) Protein lysates were prepared from long bones after removal of bone marrow (two separate age-matched WT and Kal-KO mice were used). Protein lysates were subject to SDS-PAGE and blotted for phosphorylated Erk1/2. Actin was used as the loading control. All ELISA assay experiments were reproduced at least twice and Western blot experiments were repeated 2–6 times and representative results are shown.