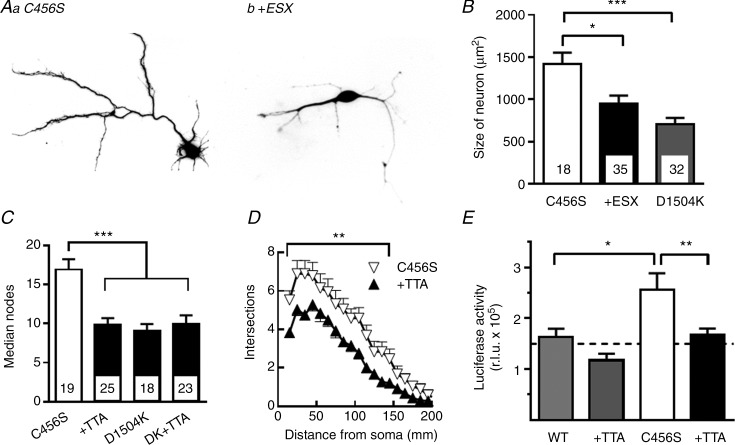
Figure 5.
A, representative images of neurons transfected with plasmids encoding mCherry and the childhood absence epilepsy variant C456S. The cells were transfected 1 day after isolation from E19 embryonic hippocampal tissue. The neurons were maintained in Neurobasal medium in the absence (Aa) and presence (Ab) of 0.3 mm ethosuximide (ESX) for 3 days before imaging. B–D, cells were analysed with Neurolucida software to determine their overall size (B), number of branch points (C) and Scholl analysis of dendritic arborization (D). D1504K is a non-conducting Cav3.2 channel variant that was used as transfection control (Supplemental Fig. 2). The data in panels C and D are from separate experiments in which the selective T-type channel antagonist, TTA-P2 (0.1 μm TTA), was used instead of ESX. E, the Gal4-transactivator trap system (Aizawa et al. 2004) was used to measure T-channel signalling to the Ca2+-activated transcription factor CREST. Hippocampal neurons were transfected with the plasmids pGL4.35, Gal4-Crest, and Syn-mGFP-2A-Cav3.2-WT or C456S, and harvested 48 h later for luciferase assay. Results refer to a representative experiment performed four times, in which statistical differences were calculated with Student's t test. The addition of TTA-P2 had no statistical effect on the luciferase activity measured in cells transfected with wild-type channels, but reversed the effect of C456S channels (n = 6 replicates). ANOVA was used for statistical analysis. Numbers of cells studied are shown inside the respective columns (B, C). Significant differences from C456S channels are shown: *P < 0.05; **P < 0.01, and ***P < 0.001.