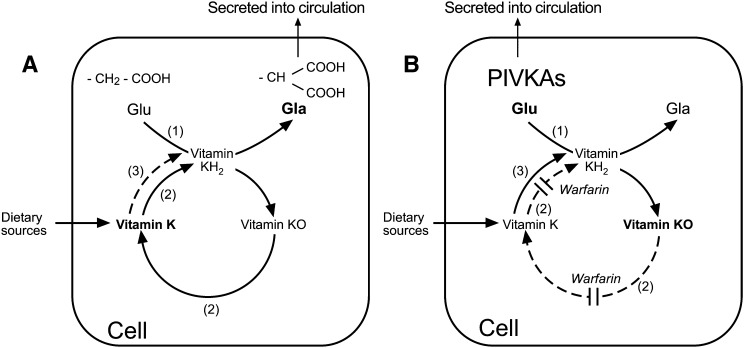
Fig. 2.
Metabolism of vitamin K via the vitamin K-epoxide cycle in the absence (A) and presence (B) of warfarin. A: In the absence of warfarin, peptide-bound glutamic acid (Glu) residues are transformed to γ-carboxy glutamic acid (Gla) residues by the enzyme γ-glutamyl carboxylase (GGCX) shown as enzyme activity (1). The active cofactor form of vitamin K required by the GGCX is the reduced form vitamin K quinol (KH2). During γ-glutamyl carboxylation, the carboxylated substrates (Gla proteins) are secreted into the circulation and KH2 becomes oxidized to vitamin K epoxide (K>O). This epoxide metabolite is reduced to vitamin K quinone by the enzyme VKOR, shown as enzyme activity (2). Vitamin K quinone is then reduced to KH2 by a vitamin K reductase activity to complete the cycle. The reduction of vitamin K quinone to KH2 may be achieved by VKOR or by a NAD(P)H-dependent activity shown as enzyme activity (3). There are several candidate quinone dehydrogenases for activity (3). B: In the presence of a VKA such as warfarin, the activity of the VKOR (2) is inhibited leading to an accumulation of K>O in the cell, and release into the circulation. As a consequence of VKOR inhibition there is a reduced capacity of the cell to generate sufficient active KH2 cofactor to enable the GGCX to carboxylate VKD peptide substrates. This results in the cellular synthesis of inactive species of undercarboxylated proteins called proteins induced by vitamin K absence or antagonism (PIVKAs), which for coagulation and bone proteins are secreted into the circulation. Given a sufficient supply of vitamin K (e.g., from the diet) the alternative quinone reductase activity (3) can bypass the warfarin inhibition of the VKOR to provide the KH2 substrate for the GGCX and hence overcome the inhibitory action of warfarin, even under extreme blockade.