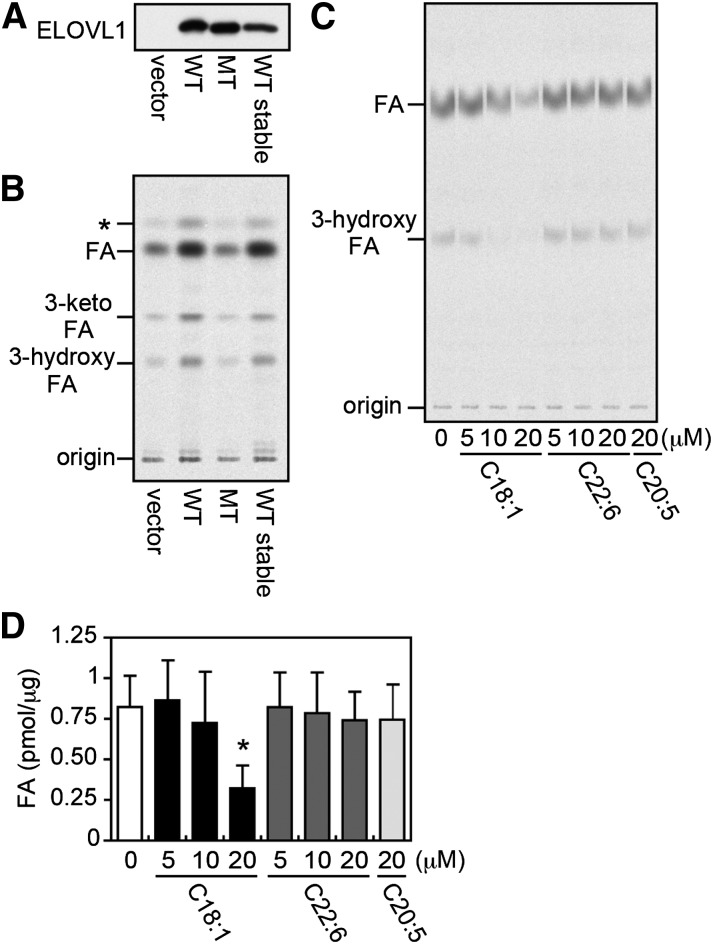
Fig. 1.
Oleic acid inhibits ELOVL1 activity. (A, B) The total membrane fraction was prepared from HeLa cells transfected with a vector (pCE-puro 3xFLAG-1), a plasmid encoding the wild-type (WT) 3xFLAG-ELOVL1 protein (pCE-puro 3xFLAG-ELOVL1), or a plasmid encoding the mutant (MT) 3xFLAG-ELOVL1 protein [pCE-puro 3xFLAG-ELOVL1(AA)], and from HeLa cells stably expressing the WT 3xFLAG-ELOVL1 protein (HeLa-ELOVL1). For transient expression, cells were harvested 24 h after transfection. (A) The total membrane fraction (5 μg protein) was subjected to immunoblotting with anti-FLAG antibody. (B) The total membrane fraction (20 μg protein) was incubated with 50 μM C22:0-CoA and 0.075 μCi (27.3 μM) [2-14C]malonyl-CoA at 37°C for 30 min. After a sequential workup (see “Materials and Methods”), lipids were separated by normal-phase TLC and detected by a bioimaging analyzer BAS-2500; *, a possible decarboxylation product of 3-keto-FA formed during saponification. (C) The in vitro FA elongation assay was performed as in (B) with the total membrane fraction (10 μg protein) prepared from HEK 293T cells transiently expressing the WT 3xFLAG-ELOVL1 protein in the presence of the indicated concentration of oleic acid (C18:1), docosahexaenoic acid (C22:6), or eicosapentaenoic acid (C20:5) in ethanol or ethanol alone (vehicle control). (D) The radioactivity associated with the product FA in (C) was quantified. Each bar represents the amount of FA generated per microgram of the total membrane protein and the mean ± SD of three independent experiments. The statistically significant difference from vehicle-treated control cells (0 μM) is indicated (* P < 0.05, Student's t-test).