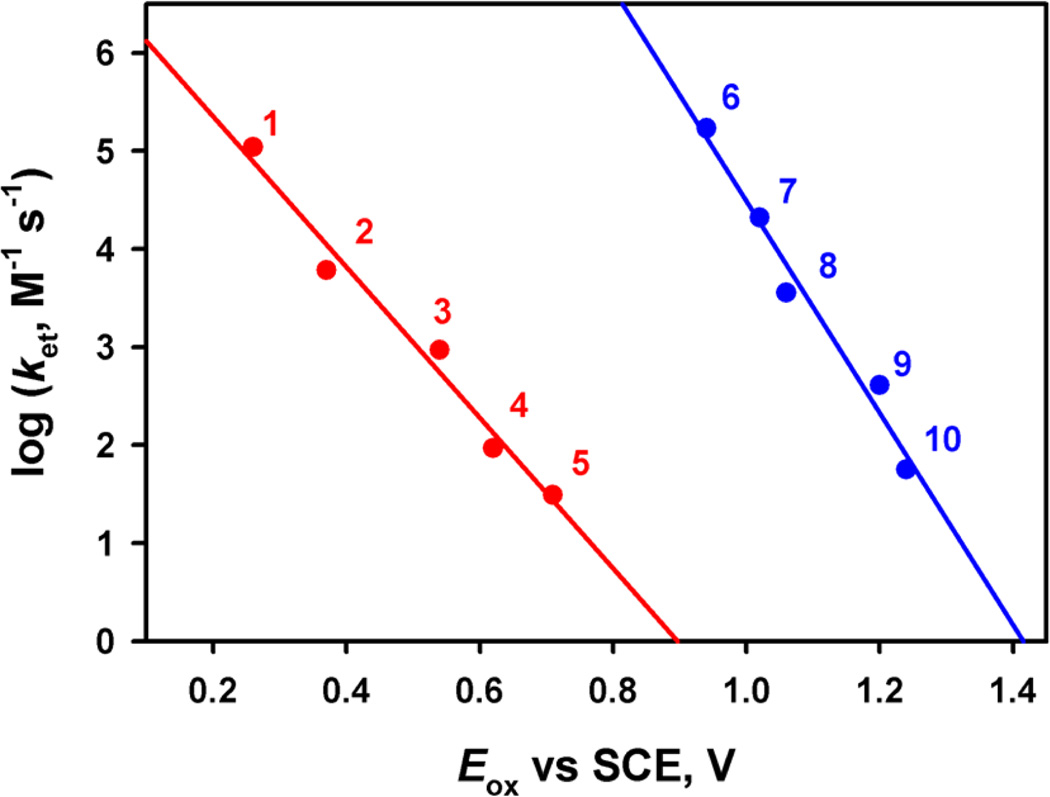
Figure 7.
Plots of log ket vs the one-electron oxidation potentials of electron donors (Eox) for electron transfer from various electron donors [(1) dimethylferrocene, (2) ferrocene, (3) bromoferrocene, (4) acetylferrocene, (5) Br2Fc, (6) [FeII(Me2-phen)3]2+, (7) [FeII(Ph2-phen)3]2+, (8) [FeII(bpy)3]2+, (9) [FeII(5-Cl-phen)3]2+, and (10) [RuII(bpy)3]2+] to [(Bn-TPEN)MnIV(O)]2+ (red line) and [(MnIV(O)]2+–(Sc3+)2 (blue line) in CF3CH2OH/CH3CN (v/v = 1:1) at 273 K.