Table 1.
Inhibition of [3H]NIC binding and [3H]MLA binding to rat brain membranes, and inhibition of nicotine-evoked [3H]DA release from superfused rat striatal slices by bis-azaaromatic quaternary ammonium analogues
| Compounda | Head group | [3H]NIC binding (% inhibition at 100 nM)b | [3H]MLA binding (% inhibition at 100 nM)b | Inhibition of nicotine-evoked [3H]DA release (% inhibition at 100 nM)b |
|---|---|---|---|---|
| bPiDDB | 0 ± 0 | 0 ± 0 | 64 | |
| 1,2-Isomers (3a) |
3aa
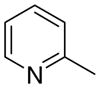
|
0 ± 0 | 7.6 ± 4.3 | 8 ± 5 |
3ab
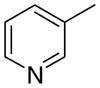
|
0 ± 0 | 5.4 ± 3.4 | 3 ± 3 | |
3ac
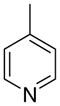
|
0 ± 0 | 3.1 ± 3.1 | 11 ± 3 | |
3ad
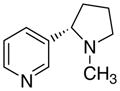
|
71 ± 2.0 | 11 ± 5.5 | 59 ± 16 | |
| 1,3-Isomers (3b) |
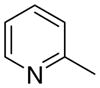
3ba
|
7.2 ± 2.8 | 0 ± 0 | 62 ± 9 |
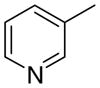
3bb
|
3.5 ± 3.5 | 6.24 ± 2.5 | 45 ± 8 | |
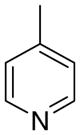
3bc
|
4.6 ± 4.0 | 3.5 ± 1.6 | 27 ± 17 | |
| 1,4-Isomers (3c) |
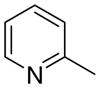
3ca
|
6.30 ± 4.21 | 0.16 ± 0.16 | 40 ± 22 |
3cb
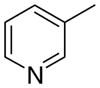
|
4.33 ± 2.19 | 7.41 ± 4.07 | 44 ± 19 | |
3cc
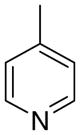
|
1.10 ± 0.89 | 7.73 ± 2.41 | 32 ± 16 | |
3cd
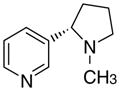
|
3.66 ± 2.39 | 5.3 ± 5.3 | 17 ± 12 | |
| 1,2-Isomers (4a) |
4aa
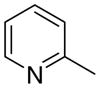
|
2.1 ± 2.1 | 1.2 ± 1.2 | 25 ± 4 |
4ab
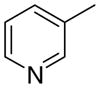
|
3.9 ± 2.8 | 0 ± 0 | 46 ± 13 | |
4ac
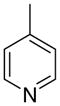
|
2.5 ± 1.5 | 5.4 ± 3.2 | 12 ± 7 | |
4ad
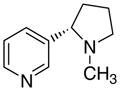
|
72 ± 3.0 | 9.2 ± 3.0 | 35 ± 20 | |
| 1,3-Isomers (4b) |
4ba
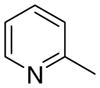
|
1.9 ± 0.7 | 0 ± 0 | 35 ± 15 |
4bb
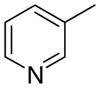
|
0.5 ± 0.4 | 8.0 ± 3.0 | 6 ± 4 | |
4bc
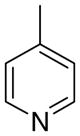
|
10 ± 5.6 | 6.4 ± 3.5 | 20 ± 12 | |
4bd
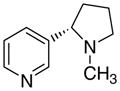
|
3.1 ± 1.7 | 2.6 ± 1.6 | 18 ± 10 | |
| 1,4-Isomers (4c) |
4cb
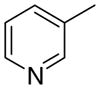
|
13.0 ± 5.4 | 3.1 ± 3.1 | 32 ± 11 |
4cc
|
8.9 ± 2.5 | 2.7 ± 2.7 | 19 ± 11 |
The first letter in the compound designation indicates orientation around the central phenyl ring (i.e., a = 1,2; b = 1,3, and c = 1, 4). The second letter indicates the nature of the head group (i.e., a = 2-picolinium, b = 3-picolinium, c = 4-picolinum, and d = nicotinium).
Each value represents data from at least three independent experiments, each performed in duplicate.
