Figure 1. Regulation of extrinsic apoptosis and RIP3 necrosis by a ‘Necrosome’ or ‘Ripoptosome’ complex.
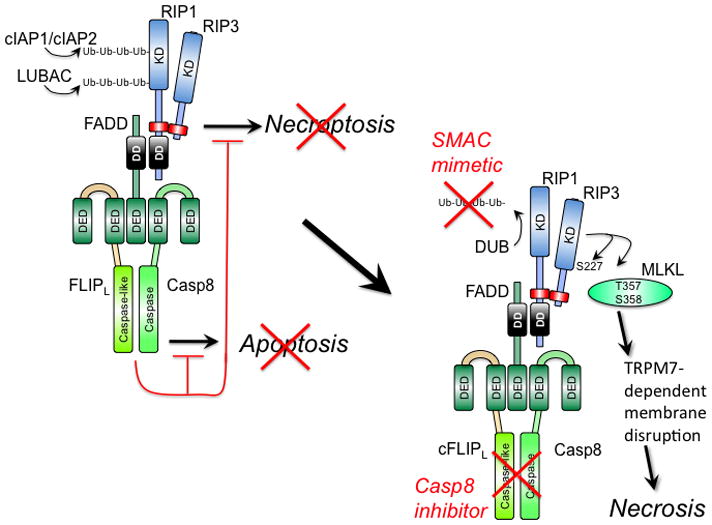
(Left) Cytoprotection. Signal transduction via death receptors (e.g. TNF) (37–39), pathogen sensors (e.g. TLR3 signaling) (27, 51), TCR activation (32, 33) or intracellular genotoxic stress (34) supports FADD association with the FLIP-Casp8 heterodimer via DED as well as RIP1 via DD interaction. RIP1 orchestrates recruitment of RIP3 via a RHIM interaction (red rectangle). The FLIP-Casp8 association prevents self-cleavage activation of Casp8 and maintains sufficient basal protease activity to also prevent necroptosis. E3 ubiquitin ligases cIAP1/cIAP2 and LUBAC also prevent necroptosis by maintaining K63 or linear polyubiquitination (Ub-Ub) of RIP1 and other targets (34, 100, 101). (Right) Activation of necroptosis. When Casp8 activity is blocked by an inhibitor or E3 ubiquitin ligases are compromised (red “X”) by a mimetic of second mitochondria-derived activator of caspases (SMAC), RIP3 kinase autophosphorylates at S277 and targets MLKL (42) for phosphorylation at T357 and S358 (41). These modifications drive trimerization of MLKL and membrane disruption associated with Ca2+ influx via a TRPM7-dependent plasma membrane channel (43). Deubiquitinase (DUB) activity removes polyubiquitin chains in the presence of SMAC mimetic, sensitizing to necrosis when Casp8 activity is compromised.
