Fig. 4. Mucosal ICMV vaccination enhances protection against tumors and pulmonary viral infection.
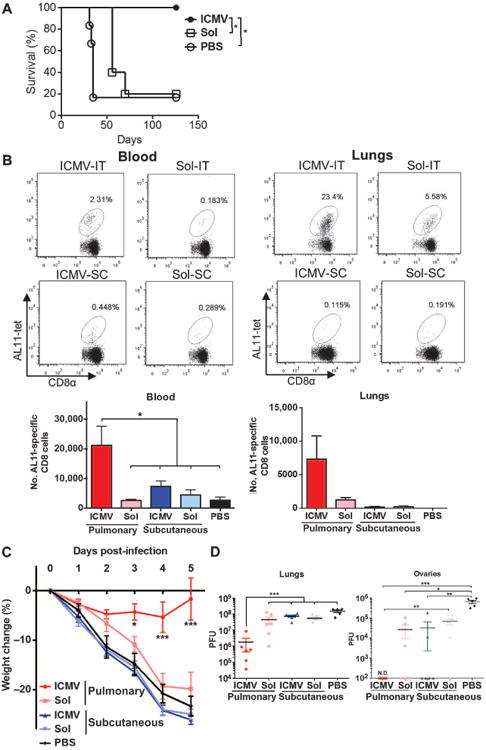
(A) C57BL/6 mice (n = 5 to 6 per group) inoculated with 5 × 105 B16F10-OVA cells subcutaneously in the flank on day 0 were treated on days 3 and 10 with intratracheal administration of ICMVs or soluble OVA vaccines. Survival of tumor-bearing mice was tracked for 18 weeks. *P < 0.05, log-rank (Mantel-Cox) test. (B) C57BL/6 mice were immunized subcutaneously (SC) or intratracheally (IT) on days 0 and 28 with AL11 and PADRE peptide ICMV or soluble vaccines. Frequencies of AL11-specific CD8+ T cells in blood and lungs were determined on day 77 by flow cytometry. (C and D) Groups of mice were immunized as in (B) and then challenged by intratracheal administration of SIV gag–expressing vaccinia virus (1 × 106 PFU) on day 42. (C) Body weight changes over time. (D) Viral titer counts in lungs and ovaries harvested 5 days after infection; dots show titers from individual animals. Data are means ± SEM with n = 3 to 7 animals per group. *P < 0.5, **P < 0.01, ***P< 0.001, by two-way ANOVA (C) or one-way ANOVA (B and D). N.D., not detectable.
