Abstract
BACKGROUND
Epoxyeicosatrienoic acids (EETs) and 20-hydroxyeicosatetraenoic acid (20-HETE) are cytochrome P450 metabolites of arachidonic acid posited to act in the circulatory adaptation to pregnancy and the development of preeclampsia. Red blood cells (RBCs) may function as major contributors of cis- and trans-EETs.
METHODS
We performed paired analyses of EETs, dihydroxyeicosatrienoic acids (DHETs), and 20-HETE in RBCs, plasma, and urine from preeclamptic and normotensive pregnant and nonpregnant women. Blood from fetal and maternal circulation was collected. EETs, DHETs, and 20-HETE were analyzed by gas chromatography and liquid chromatography mass spectrometry. Vascular function and inflammation indices were analyzed.
RESULTS
Plasma EET is higher in normotensive (median, range; 9.9, 6.3–25.2ng/mL n = 29) and preeclamptic (10.9, 6.0–48.0ng/mL, n = 19) women than in nonpregnant controls (7.3, 3.7–10.2ng/mL, n = 19) and correlate with RBC EETs, C-reactive protein, and arterial stiffness. Renal production of EETs, measured as urinary DHETs, was reduced in preeclamptic (4.5, 1.6–24.5ng/mg creatinine) compared to normotensive (11.4, 1.6–44.5ng/mg creatinine) pregnancies. EETs are 3- to 5-fold greater in fetoplacental than in maternal circulation (RBCs 36.6, 13.1–69.4 vs. 12.5, 6.4–12.0ng/109 cells; plasma 31.6, 8.5–192.6 vs. 12.0, 6.8–48.0ng/mL). Both cis- and trans-EETs are present in fetal RBCs.
CONCLUSIONS
RBCs contribute to elevated levels of EETs in the fetoplacental circulation. EETs may modulate systemic and fetoplacental hemodynamics in normal and preeclamptic pregnancies. Decreased renal EET generation may be associated with the development of maternal renal dysfunction and hypertension in preeclampsia.
Keywords: blood pressure, eicosanoids, epoxyeicosatrienoic acids, fetus, hypertension, preeclampsia, pregnancy.
Preeclampsia is a severe disorder of pregnancy characterized by the development of hypertension and proteinuria, in which increased vascular resistance and endothelial dysfunction in the mother are often accompanied by altered placental perfusion and restricted fetoplacental growth.1,2 Little information is available concerning the release of cytochrome P450 (CYP) metabolites of arachidonic acid (AA) in pregnant women.3
Epoxyeicosatrienoic acids (EETs) are involved in vasoprotective, antiinflammatory, and renal excretory mechanisms that epitomize their critical role as endogenous antipressor agents.4–6 In addition to circulating levels of EETs, EETs are formed in the placenta, trophoblast, amnion, chorion, decidua, and myometrium of the gravid uterus.7–9 EETs, acting as endothelial-derived hyperpolarizing factors, have been proposed to support uterine blood flow during preeclampsia in the face of reduced synthesis of nitric oxide and prostaglandins.2 CYP epoxygenase expression in the rat kidney is upregulated during pregnancy,10 thereby raising renal EET levels, which have been shown to antagonize prohypertensive 20-hydroxyeicosatetraenoic acid (20-HETE) in resistance blood vessels, lessening the renal vasoconstrictor response to 20-HETE.11,12 20-HETE is systemically prohypertensive, although it exerts natriuretic effects in the proximal tubules and the medullary thick ascending limb.13
CYP monooxygenase metabolism of AA produces cis-EETs and 20-HETE, while red blood cells (RBCs) generate cis- and trans-EETs from AA by activated hemoglobin and by peroxidation of AA esterified in phospholipids.14 Hemoglobin in RBCs relieves oxidative stress in the circulation through its peroxidase activity.15 EETs of cis- and trans-configuration can also be produced by the CYP interaction with lipid hydroperoxides derived from lipoxygenases.16 RBCs are reservoirs for abundant cis- and trans-EETs, which are linearly correlated with plasma EETs.17,18 Plasma EETs circulate principally esterified to the phospholipids of lipoproteins, and free EETs account for 3% or less of plasma EETs.19
EETs are hydrolyzed by the soluble epoxide hydrolase (sEH) forming dihydroxyeicosatrienoic acids (DHETs) that have generally reduced activity. The current study was designed for cross-sectional analysis by comparing plasma, RBC, and urine content of EETs, DHETs, and 20-HETE in pregnant and nonpregnant women. We hypothesize that circulatory EETs and 20-HETE are closely associated with the development of pregnancy and preeclampsia. Comparison of the levels of these eicosanoids in maternal peripheral (brachial) venous blood and urine along with levels in the fetoplacental circulation is the first step in evaluating an antipressor function for this system during pregnancy, and its contribution to the pathophysiology of preeclampsia.
METHODS
Patients
In this cross-sectional study, both normotensive and preeclamptic pregnant women were enrolled. Healthy nonpregnant women were recruited as controls. This study was performed in the Verona University Hospital and was approved by the Ethics Committee of the Hospital. All participants gave written informed consent after receiving full written information about the research project. A total number of 19 preeclamptic, 29 normotensive pregnant women, and 19 nonpregnant controls were studied, matched for age and body mass index. (See Supplementary Information for Patients and Newborns online).
Protocols
On the day of study (day 0), blood pressure, heart rate, indices of vascular function, anthropometric parameters, fasting peripheral blood (total volume approximately 20mL, anticoagulated with EDTA), and urine (from 8:00 pm day 0, to 8:00 am day 1) were collected for a biochemical profile and measurement of eicosanoids. The biochemical profile included blood cell count, plasma glucose, C-reactive protein, lipids, uric acid, and plasma and urinary creatinine, measured using automated analyzers. Creatinine clearance and urinary protein excretion were measured from 24-hour urine collection and blood obtained one day before the study (day 0).
All pregnant women who agreed to donate blood from the umbilical cord (7 preeclamptic and 14 normotensive pregnant women) were also studied at the time of surgical delivery, when samples of arterial (approximately 1mL) and venous (approximately 8mL) blood were drawn from the umbilical cord within 1–2 minutes after clamping (anticoagulated with citric acid, sodium citrate, dextrose).
RBCs and Plasma
Blood drawn from the umbilical cord artery and vein was analyzed for a biochemical profile and measurement of blood gases using automated analyzers (pO2 in umbilical artery was not measured in 6 cases due to technical reasons). RBCs were centrifuged at 800 g for 10 minutes and washed 3 times with a physiological salt solution as described.20 RBCs were counted under the microscope and diluted to 2×109 cells/mL. As an antioxidant, 0.1mM triphenylphosphine was added to RBC and plasma samples and stored at –70°C until eicosanoid analysis.
Quantification of Eicosanoids
Plasma-free and total EETs, DHETs, and 20-HETE were extracted with ethyl acetate as in previous studies, except that the internal standards used for DHETs were 14,15- and 8,9-DHET-d11 (2ng each).14,19 RBC phospholipid extraction was carried out using Rose and Oklander’s method after the addition of internal standards.14,21 Purified eicosanoids were derivatized and subjected to gas chromatography mass spectrometry (GC/MS) analysis as described.14,19 Some of the human plasma and RBC samples were also analyzed by liquid chromatography coupled with tandem mass spectrometry (LC/MS/MS) (n = 10) to confirm the presence of cis-/trans-EETs and threo-/erythro-DHETs as described.17,22
Blood Pressure and Vascular Function
Blood pressure was measured twice at the time of enrollment with a sphygmomanometer by a trained nurse, whereas during vascular examination, it was measured using an oscillometric device (A&D Instruments) with the subjects in the supine position after 10 minutes’ rest. The stiffness index (SI) and reflection index (RI) were analyzed for structural and functional characteristics of arterial circulation. SI, an index of vascular compliance, and RI, an index of artery resistances, were measured by a photoplethysmograph (Micro Medical) placed on the index finger of the right hand used to obtain the digital volume pulse.23
Statistical Analysis
Data are expressed as mean and SEM or the median and range as indicated. PRISM 4 and SPSS-20 statistic package were used for the analysis. Analysis of variance was applied when normal distribution of the data was identified using the D’Agostino test (biochemical profile and hemodynamic parameters); the Newman–Kleus test was used for post hoc analysis. The Kruskal–Wallis test was used for multiple comparisons when normal distribution was not demonstrated. The Dunn test was applied to post hoc pairwise comparisons. The Mann–Whitney test was applied when two unpaired variables were compared. The Pearson coefficient (r) was calculated to quantify correlation between variables. Multiple linear regression analysis was used in the multivariate model with EETs as dependent variables and age, mean arterial pressure, SI, and C-reactive protein as independent variables. P < 0.05 was considered statistically significant.
RESULTS
Cross-sectional Comparison of Pregnant and Nonpregnant Women and Newborn From Preeclamptic and Normotensive Pregnancies
The characteristics of preeclamptic and normotensive pregnancies and age-matched pregnant women at the time of delivery are indicated in Table 1.
Table 1.
Clinical indices of pregnancies
| Variable | Preeclampsia (n = 19) | Normal pregnancy (n = 29) | Nonpregnant controls (n = 19) | ANOVA P values |
|---|---|---|---|---|
| Age, years1 | 34 (21–40) | 33 (26–40) | 31 (24–45) | P = 0.22 |
| Pregnancy, weeks1 | 33 (25–40)2 | 38 (24–40) | – | – |
| Systolic pressure, mmHg3 | 139±34,5 | 111±2 | 111±2 | P < 0.0001 |
| Diastolic pressure, mmHg3 | 87±34,5 | 72±1 | 69±1 | P < 0.0001 |
| Body mass index, kg/m2 | 21.5±0.6 | 22.5±0.5 | 22.4±0.6 | P = 0.46 |
| Stiffness index, m/s | 8.4±0.62,6 | 7.2±0.2 | 6.9±0.3 | P = 0.01 |
| Reflection index, % | 64.7±2.84 | 51.4±1.75 | 61.5±1.8 | P < 0.0001 |
| Proteinuria (24h), mg1 | 1300 (300–15000)4,5 | 45 (10–150) | 25 (10–75) | P < 0.0001 |
| Hematocrit, % | 34.4±0.85 | 34.4±0.65 | 39.7±0.5 | P < 0.0001 |
| Platelets, cells/mm3 | 240,000(133,000–476,000)6 | 276,000(88,000–458,000) | 309,000(216,000–437,000) | P < 0.01 |
| C-reactive protein, mg/L | 4.21±1.105 | 3.16±0.536 | 0.96±0.17 | P < 0.01 |
| Plasma creatinine, mg/dL | 0.70±0.024 | 0.55±0.025 | 0.70±0.02 | P < 0.0001 |
| Creatinine clearance, mL/min | 108.8±5.84 | 141.2±8.36 | 114.8±5.7 | P = 0.01 |
| Plasma uric acid, mg/dL | 5.9±0.34,5 | 4.1±0.2 | 3.9±0.2 | P < 0.0001 |
| Plasma glucose, mg/dL | 86.0±4.1 | 77.9±1.86 | 86.8±1.3 | P < 0.05 |
| Plasma cholesterol, mg/dL | 262.4±14.45 | 276.2±12.25 | 183.5±8.7 | P < 0.0001 |
| Plasma triglycerides, mg/dL | 277.6±22.05 | 225.9±16.55 | 65.5±5.2 | P < 0.0001 |
Data are mean ± SEM, unless otherwise noted.
1Data are median (range); P < 0.05 vs. 2normal pregnancy or vs. 6control.
3Blood pressure values of preeclamptic patients are those recorded at the time of delivery after pharmacological intervention. The values on which diagnosis of preeclampsia was based were higher at the time of hospital admission: systolic blood pressure 155 (140–170) mmHg, diastolic blood pressure 100 (90–110 mmHg).
P < 0.01 vs. 4normal pregnancy or vs. 5controls.
Abbreviation: ANOVA, analysis of variance.
Blood pressure levels were still significantly higher than in normal pregnancy and nonpregnant controls, despite antihypertensive therapy. Serum creatinine and uric acid levels were elevated, while the creatinine clearance was reduced in preeclamptic women compared to normal pregnancy. As expected, plasma lipids were higher in pregnant women (Table 1).
The reflection index was lower in women with normal pregnancy compared to both nonpregnant controls and preeclamptic women, indicating a reduction in peripheral vascular resistance in normal pregnancies. Arterial stiffness, as assessed by measuring the SI, was elevated in preeclamptic women but not in normotensive pregnant and nonpregnant women (Table 1).
The weight of the newborn and the placenta of preeclamptic women were lower than the expected fetoplacental weight in normal pregnancy of corresponding gestational age (5 cases were below the 5th percentile; Supplementary Information Table 1).
EETs and DHETs in RBCs, Plasma, and Urine of Preeclamptic and Normotensive Pregnancy in Comparison With Nonpregnant Controls
Pregnant women, preeclamptic and normotensive, had higher peripheral blood plasma EETs than nonpregnant controls (Figure 1A). No differences in RBC and plasma DHET concentrations were observed between pregnant and non-pregnant women (Figure 1B and Supplementary Information Figure 1A,B).
Figure 1.
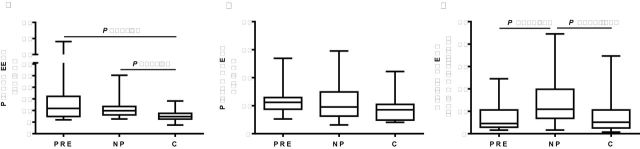
Comparison of CYP eicosanoid levels among preeclampsia (PRE), normal pregnancy (NP), and nonpregnant controls (C). (a) Plasma EETs. (b) Plasma DHETs. (c) Urinary DHET over creatinine excretion. Values of median and range are shown (PRE, n = 19; NP, n = 29; and C, n = 19). Abbreviations: CYP, cytochrome P450; DHETs, dihydroxyeicosatrienoic acids; EETs, epoxyeicosatrienoic acids.
The urinary excretion of DHETs, which was the surrogate for EET excretion, increased in normotensive pregnant women when compared with nonpregnant women (Figure 1C). However, excretion of DHETs in preeclamptic pregnancies was reduced to half of the DHET excretion in normotensive pregnancies (Figure 1C). Consistent with our previous study,19 EETs in human urine could not be detected.
No significant difference was identified in EET, DHET, or 20-HETE levels in RBCs, plasma, and urine between gestational age at 24–34 weeks and 35–40 weeks for preeclamptic and normotensive pregnancies (data not shown). RBC EETs correlated significantly with plasma total EETs (r = 0.45, P < 0.001; Supplementary Information Figure 2A).
To help understand the role of eicosanoids in pregnancy and preeclampsia, correlations among the major endpoints of the study were calculated. Brachial venous plasma EETs were positively correlated with plasma C-reactive protein in preeclamptic and normotensive pregnancies (Figure 2A). Correlations were also found between brachial venous plasma EETs and SI (Figure 2B), as well as mean arterial pressure in preeclamptic and normotensive pregnancies (Figure 2C). A strict correlation between SI and plasma EETs was found when only preeclamptic women were included (preeclamptic women: plasma EETs-SI: r = 0.68, P < 0.005, n = 15). Multivariate regression analysis, including the mentioned covariates, showed SI as the predictor for EET levels in plasma, when preeclamptic women were considered (SI: β ± SEM, 1.171±0.502, P < 0.05; C-reactive protein: 0.355±0.258, P = 0.16; mean arterial pressure: –0.008±0.117, P = 0.94; constant: 2.289±4.689, P = 0.63). C-reactive protein was also an independent predictor for EET levels in plasma when all pregnant women were included in the multivariate regression analysis (SI: β ± SEM, 0.825±0.38, P < 0.05; C-reactive protein: 0.497±0.168, P < 0.005; mean arterial pressure: 0.011±0.059, P = 0.85; constant: 2,105±11,363, P = 0.86). Multicollinearity was not found.
Figure 2.
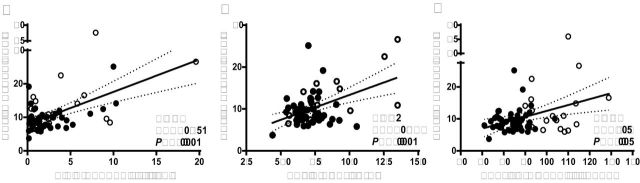
Correlation of brachial venous plasma EETs from preeclamptic and normotensive pregnancies with C-reactive protein (a), stiffness index (b), and mean blood pressure (c). The best-fit line and 95% confidence bands are shown. Open circles indicate preeclamptic and solid circles indicate normotensive pregnancies. Abbreviation: EETs, epoxyeicosatrienoic acids.
Comparative Analysis of Maternal and Fetal Blood
Consistent with the low oxygen tension that characterizes the fetoplacental circulation,24 the analysis of blood from the umbilical cord revealed low pO2 values not only in arteries carrying blood from the fetus to the placenta (21.2±2.0 mmHg), but also in veins (27.4±1.9 mmHg, P < 0.01 vs. arteries). In newborns, higher pCO2 values were found in umbilical arteries (55.7±3.8 mmHg) than in umbilical veins (49.5±1.3 mmHg, P < 0.001). Lower pH and higher pCO2 values were recorded in newborns of preeclamptic women compared to normal pregnancies (See Supplementary Information for Patients and Newborns online).
Comparison of maternal brachial vis a vis umbilical cord venous content of EETs and DHETs in RBCs and plasma showed that RBC EET concentrations in the umbilical cord vein were 2- to 3-fold higher than in the brachial vein in normotensive and preeclamptic pregnancies (Figure 3A). However, RBC EETs in the umbilical cord did not differ on comparison of preeclamptic with normotensive pregnancies (Supplementary Information Table 2), which was also the case for RBC EETs in brachial venous blood (Supplementary Information Figure 2A). Preeclamptic and normotensive pregnancies all had 4-fold higher umbilical vein than brachial vein plasma EET levels (Figure 3B) with no differences between preeclamptic and normotensive pregnancies (Supplementary Information Table 2). The levels of DHETs in umbilical vein RBCs and plasma in both preeclamptic and normotensive pregnancies were 2-fold higher than in the brachial vein (Figure 3C, D and Supplementary Table S2 online).
Figure 3.
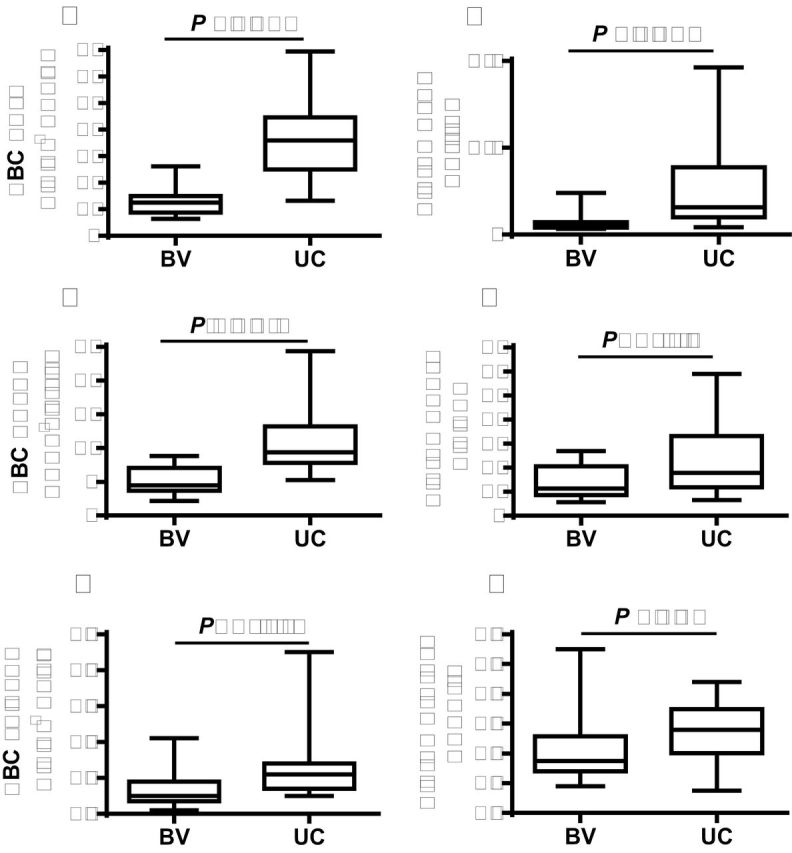
Comparison between brachial venous (BV) and umbilical cord (UC) venous blood levels of CYP eicosanoids in all (combining preeclamptic and normotensive) pregnancies. (a) RBC EETs. (b) Plasma EETs. (c) RBC DHETs. (d) Plasma DHETs. (e) RBC 20-HETE. (f) Plasma 20-HETE. Values of median and range are shown (n = 21). Abbreviations: 20-HETE, 20-hydroxyeicosatetraenoic acid; CYP, cytochrome P450; DHETs, dihydroxyeicosatrienoic acids; EETs, epoxyeicosatrienoic acids; RBC, red blood cell.
Exploratory analysis showed that fetal pCO2, a marker of hypoxemia, correlated positively with fetoplacental plasma EETs (Supplementary Information Figure 2B). Correlation was closer when only preeclamptic pregnancies were considered (r = 0.76, P < 0.05). Placental weight correlated negatively with fetoplacental plasma EETs when preeclamptic and normotensive pregnancies were considered (r = –0.44; P < 0.05; see Supplementary Figure 2 online).
20-HETE in Maternal and Fetal Blood and in Urine
20-HETE concentrations in RBCs in both preeclamptic and normotensive pregnancies were vanishingly low (Table 2 and Figure 3E), falling below 0.15ng/109 cells in contrast to RBC EETs of as much as 200-fold greater (Table 2 and Figure 3).
Table 2.
Levels of 20-HETE in plasma, RBCs, and urine in preeclamptic, normotensive pregnant and nonpregnant women
| Variable | Preeclampsia (n = 19) | Normal pregnancy (n = 29) | Nonpregnant controls (n = 19) | ANOVA P values |
|---|---|---|---|---|
| Plasma 20-HETE, ng/mL | 0.48±0.06 | 0.40±0.03 | 0.51±0.03 | P = 0.06 |
| RBC 20-HETE, ng/109 cells | 0.07±0.02 | 0.10±0.02 | 0.13±0.03 | P = 0.27 |
| Urinary 20-HETE, ng/mg creatinine | 0.73±0.15 | 1.46±0.40 | 1.17±0.36 | P = 0.37 |
Plasma and RBCs were from brachial venous blood. Data are mean ± SEM.
Abbreviations: 20-HETE, 20-hydroxyeicosatetraenoic acid; ANOVA, analysis of variance; RBCs, red blood cells.
There were no differences in plasma 20-HETE levels between preeclamptic and normotensive pregnancies, either in umbilical cord or in maternal blood (Table 2), although levels of plasma 20-HETE were generally higher in the umbilical cord than in the maternal blood (Figure 3F). Urinary excretion of 20-HETE in nonpregnant controls, normotensive, and preeclamptic pregnancies did not show a statistical difference (Table 2).
The Chemical Identities of EETs and DHETs
The GC/MS analysis revealed the presence of both cis- and trans-EETs as well as their hydration products, threo- and erythro-DHETs, respectively, in RBCs and in plasma, which were compared to authentic standards in Figure 4. In GC/MS chromatograms, 14,15- and 11,12-trans-EET coeluted (tr 4.53min) ahead of the major peak for EETs (tr 4.58min) that represents an overlap of the other 6 EETs (Figure 4A). GC/MS analysis of DHETs revealed 3 peaks, the first peak (tr 4.47min) representing 11,12-, 8,9- and 5,6-threo-DHETs, the middle one (tr 4.52min) representing 14,15-threo-DHET and 11,12-, 8,9- and 5,6-erythro-DHETs, and the last peak (tr 4.58min) standing only for 14,15-erythro-DHET (Figure 4B).
Figure 4.
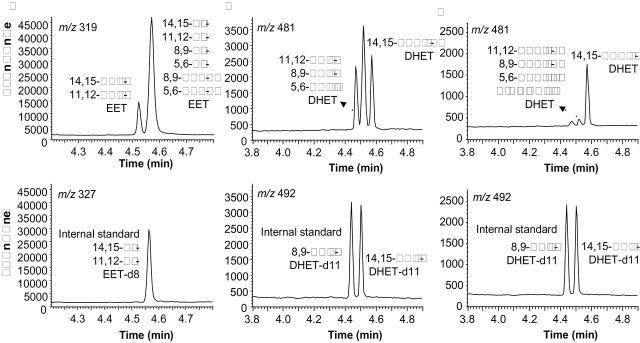
Presence of cis- and trans-EETs and threo- and erythro-DHETs in human plasma and RBCs as identified by GC/MS analyses. Representative GC/MS chromatograms are shown for: (a) RBC and plasma EETs; (b) RBC and plasma total DHETs; (c) Plasma-free DHETs. Identities of endogenous (top) and internal standards (bottom) are labeled beside respective peaks. Abbreviations: DHETs, dihydroxyeicosatrienoic acids; EETs, epoxyeicosatrienoic acids; GC/MS, gas chromatography mass spectrometry; RBC, red blood cell.
Notably, 14,15-erythro-DHET, the hydrolysis product of 14,15-trans-EET, stands out as the major free DHET peak, constituting over 80% of free DHETs in human plasma (Figure 4C), which is consistent with the finding that 14,15-trans-EET is the most rapidly hydrolyzed EET by RBCs.
These EET and DHET isomers in RBCs and in plasma were confirmed by LC/MS/MS analysis using established methods (Supplementary Information Figure 3A–C). The ratio of cis-/trans-EETs in human RBCs was similar (A: ratio 2.01±0.07, range 1.55–2.30, n = 10), while the ratio of cis-/trans-EETs in human plasma varied broadly (B-C: ratio 2.87±0.49, range 0.93–5.11, n = 10), possibly reflecting a different mechanism for trans-EET formation.
DISCUSSION
Major findings from the present study were: (i) more than 3-fold higher levels of EETs in fetal than in maternal blood; (ii) increased plasma levels of EETs in both preeclamptic and normotensive pregnancy compared to nonpregnant women; and (iii) reduced urinary excretion of EETs in the form of DHETs in preeclamptic than normotensive pregnancy. Unanswered questions in this study are the individual quantification of EET and DHET isomers and their identity as potential pathophysiological markers or contributors to the hemodynamic alterations of complicated and normotensive pregnancy. The role of trans-EETs as well as their products of sEH, erythro-DHETs, in promoting circulatory homeostasis in preeclampsia needs further clarification. Increased levels of 5/12/15-HETEs associated with EETs is a distinct possibility in preeclampsia as a result of the hemoglobin peroxidase activity that breaks down lipid hydroperoxides and generates cis- and trans-EETs.3,16
In both normal and preeclamptic pregnancies, the levels of EETs in RBCs and plasma of umbilical cord blood draining the fetoplacental circulation were much higher than EET levels in brachial venous blood, which conveys the magnitude of the reservoir function of RBCs and identifies RBCs as the likely principal carrier of EETs circulating in plasma primarily acylated to RBC phospholipids, whereas 3% or less of EETs circulate in the free form. Elevated plasma EETs may arise from increased CYP-derived metabolism of AA in the fetoplacental unit. The present study supports the hypothesis that the fetoplacental unit contributes to increased plasma levels of EETs and DHETs in pregnant women. The commanding levels of EETs in umbilical vein RBCs and plasma suggest a function of erythrocytes in the regulation of the fetal circulation and fetal development based on antipressor, anti-inflammatory, and angiogenic activities amongst other properties of EETs,4–6 though such a role for the developing fetus has yet to be addressed.
The higher levels of EETs in the fetoplacental circulation than in the maternal circulation may also reflect the greater peroxidase activity of fetal hemoglobin. Fetal erythrocytes obtained from umbilical venous blood exhibited 3–5 times more monooxygenase activity than erythrocytes obtained from the adult peripheral circulation.25 The principal presence of 14,15-erythro-DHET in plasma (Figure 4C) reflects the preferential hydrolysis of 14,15-trans-EET by RBC sEH followed by the rapid release of 14,15-erythro-DHET from RBCs.22 Thus, EETs through their anti-inflammatory, vasoactive, and thrombolytic activities represent a homeostatic system in which RBCs, in addition to delivering oxygen, participate in circulatory regulation through release of lipid mediators.
EETs and 20-HETE are critical components of both renal tubular transport and renal vascular mechanisms that control blood pressure. DHET urinary excretion, which was used as an index of renal EET production, showed a significant decline in preeclamptic pregnancies, whereas a sharp rise was seen in DHET urinary excretion in normal pregnancy, more than 2-fold above levels in nonpregnant women. As intrarenal rather than systemic EETs has been reported as the origin of urinary DHETs,26 the decline in DHET excretion in preeclampsia can be interpreted as evidence of deficient renal EET formation in preeclamptic pregnancy. The inability of preeclamptic women to activate renal EET production may contribute to the elevation of blood pressure and recalls the development of hypertension in the Dahl salt-sensitive (DS) rat produced by its inability to mobilize renal EET production.
Catella and colleagues have reported that in preeclampsia the excretion of DHETs increased to a greater degree than in healthy pregnant women.26 However, direct comparison of this study with the present study is complicated by the severity of hypertension and clinical conditions of patients in the study by Catella et al. (mean blood pressure 182/112 mmHg, reduced platelet count).
A positive correlation between total EETs and arterial stiffness, particularly in preeclamptic women, as well as C-reactive protein in maternal plasma, suggest that vascular alterations and inflammation may trigger EET generation, which is consistent with the finding that lipid hydroperoxides stimulate EET formation.16 C-reactive protein is increased during pregnancy as a result of the adaptation of the innate immune system, and may represent a biomarker of inflammation in preeclampsia.27,28 SI and RI are increased in preeclampsia, while are similar at the third trimester for normotensive pregnancy compared to those observed in nonpregnant women, consistent with previous observations.29
EETs may participate in the adaptation to low oxygen tension that characterizes fetoplacental circulation and ischemia.24 Indeed, plasma EETs are positively correlated with umbilical venous blood pCO2 draining the fetal circulation, a clinical marker for fetal tissue hypoxia. Expression of hypoxia-inducible factor-responsive genes is also linked to the induction of CYP epoxygenases EETs play an important role in cellular hypoxic and ischemic responses by stimulating production of vascular endothelial growth factor and erythropoietin.30 EETs should assume greater importance as vasoactive mediators acting as endothelial-derived hyperpolarizing factors with the decline in nitric oxide caused by free-radical scavenging and in response to reduced production of vascular nitric oxide and prostacyclin.9
Due to the cross-sectional design of the study, cause–effect relationships that require further mechanistic and interventional studies could not be elucidated. Without inclusion of an essential control group with uncomplicated chronic hypertension who do not develop preeclampsia, the study may be limited in claiming a role of EETs in the development of preeclampsia; however, essential hypertension alone had no difference in urinary EET excretion and circulatory EET levels compared to controls.19 Having only included patients with uncomplicated, although in most cases, severe preeclampsia, subgroup analysis of mild–moderate vs. severe preeclampsia could not be performed. Finally, advanced LC/MS/MS analysis is needed for the quantification of individual EET and DHET isomers in the circulation.
In conclusion, the antipressor, antiinflammatory, antiapoptotic, and angiogenic activities of EETs may represent the basis for cellular protective actions in the fetoplacental unit and vascular homeostasis in pregnancy, which is highlighted by 3 to 5-fold greater EET levels in fetoplacental than in maternal circulation and significantly elevated levels of circulatory EETs in pregnant compared to nonpregnant women. Reduced intrarenal generation of EETs, as reflected by decreased urinary excretion of DHETs, may contribute to development of hypertension in preeclampsia.
SUPPLEMENTARY MATERIAL
Supplementary materials are available at the American Journal of Hypertension online (http://www.oxfordjournals.org/our_journals/ajh/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
DISCLOSURE
The authors declared no conflict of interest.
Supplementary Material
ACKNOWLEDGMENTS
We thank Alessandra Meneguzzi for her technical support in sample collection, Kavita Jain, Samantha Benjamin, and Erik Trexler for sample analysis. Gail Anderson prepared the manuscript, for which we are grateful.
REFERENCE
- 1. Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet 2005; 365: 785–99 [DOI] [PubMed] [Google Scholar]
- 2. Taylor RN, Davidge ST, Roberts JM. Endothelial cell dysfunction and oxidative stress. In Marshall DL, James MR, Cunningham FG, (eds), Chesley’s Hypertensive Disorders in Pregnancy. 2nd edn San Diego: Academic Press; 2009, pp. 143–67 [Google Scholar]
- 3. Pearson T, Zhang J, Arya P, Warren AY, Ortori C, Fakis A, Khan RN, Barrett DA. Measurement of vasoactive metabolites (hydroxyeicosatetraenoic and epoxyeicosatrienoic acids) in uterine tissues of normal and compromised human pregnancy. J Hypertens 2010; 28: 2429–37 [DOI] [PubMed] [Google Scholar]
- 4. Campbell WB, Fleming I. Epoxyeicosatrienoic acids and endothelium-dependent responses. Pflugers Arch 2010; 459: 881–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nithipatikom K, Gross GJ. Review article: epoxyeicosatrienoic acids: novel mediators of cardioprotection. J Cardiovasc Pharmacol Ther 2010; 15: 112–19 [DOI] [PubMed] [Google Scholar]
- 6. Elmarakby AA. Reno-protective mechanisms of epoxyeicosatrienoic acids in cardiovascular disease. Am J Physiol Regul Integr Comp Physiol 2012; 302: R321–30 [DOI] [PubMed] [Google Scholar]
- 7. Schaefer WR, Werner K, Schweer H, Schneider J, Arbogast E, Zahradnik HP. Cytochrome P450 metabolites of arachidonic acid in human placenta. Prostaglandins 1997; 54: 677–87 [DOI] [PubMed] [Google Scholar]
- 8. Patel L, Sullivan MH, Elder MG. Production of epoxygenase metabolite by human reproductive tissues. Prostaglandins 1989; 38: 615–24 [DOI] [PubMed] [Google Scholar]
- 9. Gerber RT, Anwar MA, Poston L. Enhanced acetylcholine induced relaxation in small mesenteric arteries from pregnant rats: an important role for endothelium-derived hyperpolarizing factor (EDHF). Br J Pharmacol 1998; 125: 455–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Huang H, Chang HH, Xu Y, Reddy DS, Du J, Zhou Y, Dong Z, Falck JR, Wang MH. Epoxyeicosatrienoic acid inhibition alters renal hemodynamics during pregnancy. Exp Biol Med (Maywood ) 2006; 231: 1744–52 [DOI] [PubMed] [Google Scholar]
- 11. Llinas MT, Alexander BT, Capparelli MF, Carroll MA, Granger JP. Cytochrome P-450 inhibition attenuates hypertension induced by reductions in uterine perfusion pressure in pregnant rats. Hypertension 2004; 43: 623–28 [DOI] [PubMed] [Google Scholar]
- 12. Wang MH, Zand BA, Nasjletti A, Laniado-Schwartzman M. Renal 20-hydroxyeicosatetraenoic acid synthesis during pregnancy. Am J Physiol Regul Integr Comp Physiol 2002; 282: R383–89 [DOI] [PubMed] [Google Scholar]
- 13. Williams JM, Murphy S, Burke M, Roman RJ. 20-hydroxyeicosatetraeonic acid: a new target for the treatment of hypertension. J Cardiovasc Pharmacol 2010; 56: 336–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jiang H, McGiff JC, Quilley J, Sacerdoti D, Reddy LM, Falck JR, Zhang F, Lerea KM, Wong PY. Identification of 5,6-trans-epoxyeicosatrienoic acid in the phospholipids of red blood cells. J Biol Chem 2004; 279: 36412–418 [DOI] [PubMed] [Google Scholar]
- 15. Widmer CC, Pereira CP, Gehrig P, Vallelian F, Schoedon G, Buehler PW, Schaer DJ. Hemoglobin can attenuate hydrogen peroxide-induced oxidative stress by acting as an antioxidative peroxidase. Antioxid Redox Signal 2010; 12: 185–98 [DOI] [PubMed] [Google Scholar]
- 16. Jiang H, Harrison FE, Jain K, Benjamin S, May JM, Graves JP, Zeldin DC, Falck J, Hammock BD, McGiff JC. Vitamin C activation of the biosynthesis of epoxyeicosatrienoic acids. Adv Biosci Biotechnol 2012; 3: 204–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jiang H, Quilley J, Reddy LM, Falck JR, Wong PY, McGiff JC. Red blood cells: reservoirs of cis- and trans-epoxyeicosatrienoic acids. Prostaglandins Other Lipid Mediat 2005; 75: 65–78 [DOI] [PubMed] [Google Scholar]
- 18. Goulitquer S, Dreano Y, Berthou F, Corcos L, Lucas D. Determination of epoxyeicosatrienoic acids in human red blood cells and plasma by GC/MS in the NICI mode. J Chromatogr B Analyt Technol Biomed Life Sci 2008; 876: 83–88 [DOI] [PubMed] [Google Scholar]
- 19. Minuz P, Jiang H, Fava C, Turolo L, Tacconelli S, Ricci M, Patrignani P, Morganti A, Lechi A, McGiff JC. Altered release of cytochrome p450 metabolites of arachidonic acid in renovascular disease. Hypertension 2008; 51: 1379–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jiang H, Zhu AG, Mamczur M, Falck JR, Lerea KM, McGiff JC. Stimulation of rat erythrocyte P2X(7) receptor induces the release of epoxyeicosatrienoic acids. Br J Pharmacol 2007; 151: 1033–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rose HG, Oklander M. Improved procedure for the extraction of lipids from human erythrocytes. J Lipid Res 1965; 63: 428–31 [PubMed] [Google Scholar]
- 22. Jiang H, Zhu AG, Mamczur M, Morisseau C, Hammock BD, Falck JR, McGiff JC. Hydrolysis of cis- and trans-epoxyeicosatrienoic acids by rat red blood cells. J Pharmacol Exp Ther 2008; 326: 330–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Millasseau SC, Kelly RP, Ritter JM, Chowienczyk PJ. Determination of age-related increases in large artery stiffness by digital pulse contour analysis. Clin Sci (Lond) 2002; 103:371–7 [DOI] [PubMed] [Google Scholar]
- 24. Salafia CM, Minior VK, Lopez-Zeno JA, Whittington SS, Pezzullo JC, Vintzileos AM. Relationship between placental histologic features and umbilical cord blood gases in preterm gestations. Am J Obstet Gynecol 1995; 173: 1058–64 [DOI] [PubMed] [Google Scholar]
- 25. Blisard KS, Mieyal JJ. Differential monooxygenase-like activity of fetal and adult erythrocytes. Biochem Biophys Res Commun 1980; 96: 1261–66 [DOI] [PubMed] [Google Scholar]
- 26. Catella F, Lawson JA, Fitzgerald DJ, FitzGerald GA. Endogenous biosynthesis of arachidonic acid epoxides in humans: increased formation in pregnancy-induced hypertension. Proc Natl Acad Sci USA 1990; 87: 5893–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Myatt L, Webster RP. Vascular biology of preeclampsia. J Thromb Haemost 2009; 7: 375–84 [DOI] [PubMed] [Google Scholar]
- 28. Versen-Hoeynck FM, Hubel CA, Gallaher MJ, Gammill HS, Powers RW. Plasma levels of inflammatory markers neopterin, sialic acid, and C-reactive protein in pregnancy and preeclampsia. Am J Hypertens 2009; 22: 687–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kaihura C, Savvidou MD, Anderson JM, McEniery CM, Nicolaides KH. Maternal arterial stiffness in pregnancies affected by preeclampsia. Am J Physiol Heart Circ Physiol 2009; 297: H759–64 [DOI] [PubMed] [Google Scholar]
- 30. Suzuki S, Oguro A, Osada-Oka M, Funae Y, Imaoka S. Epoxyeicosatrie noic acids and/or their metabolites promote hypoxic response of cells. J Pharmacol Sci 2008; 108: 79–88 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


