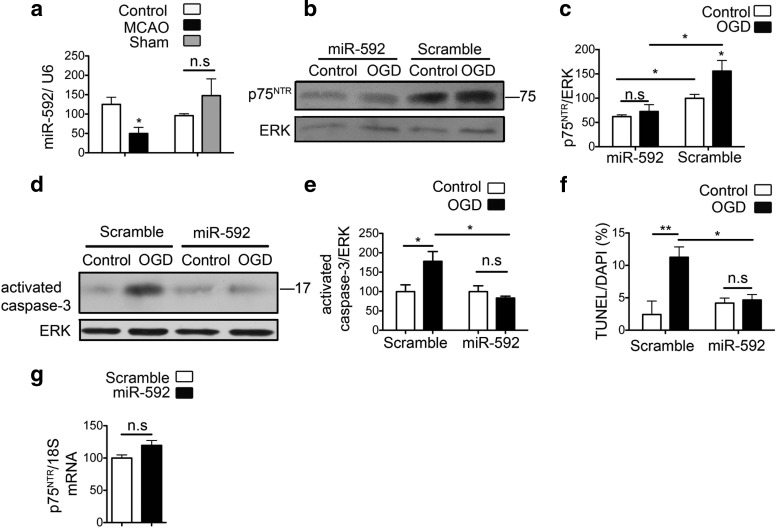
Figure 6.
MiR-592 regulates induction of p75NTR after ischemic injury in neurons and contributes to apoptosis. a, Quantitative real-time PCR shows decreased levels of miR-592 in the ischemic cortex of mice subjected to MCAO compared with the contralateral hemisphere. Sham surgeries do not affect the levels of miR-592. MicroRNA levels are normalized to endogenous small RNA U6 (n = 4). Student's t test was used to compare the miRNA levels between MCAO or sham with their respective contralateral control hemispheres. b, Primary hippocampal neurons were transfected with precursor miR-592 or a control vector and subjected to 60 min of OGD. Transfection with miR-592 decreases the levels of p75NTR under control conditions and also mitigates the OGD-induced increase in p75NTR levels. c, Quantification of p75NTR from b (n = 4; ANOVA). d, Activation of capsase-3 is observed at 8 h after OGD in primary hippocampal neurons. Overexpression of miR-592, but not the control scramble microRNA, significantly attenuates the OGD-induced activation of capsase-3. e, Quantification of activated caspase-3 from d (n = 5; ANOVA). f, Quantification of apoptotic cells after transduction with SCR or miR-592 in primary hippocampal neurons. TUNEL assay quantification shows decreased apoptosis in the presence of miR-592. An average of six fields were analyzed per coverslip, and values are shown as a percentage of TUNEL-positive cells to DAPI (n = 3; ANOVA). g, Transfection of precursor miR-592 in primary neurons does not affect the levels of p75NTR mRNA. p75NTR mRNA levels are normalized to endogenous 18S RNA (n = 4; Student's t test). *p < 0.05; **p < 0.001. n.s., Not significant.