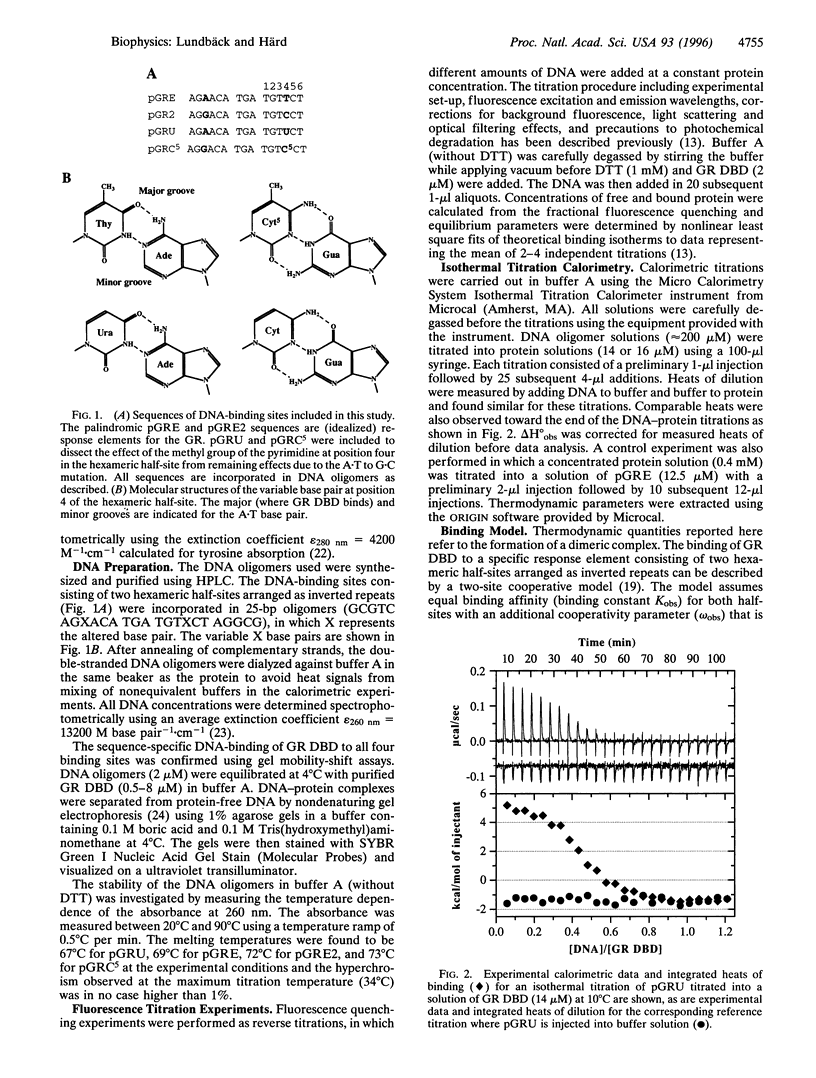
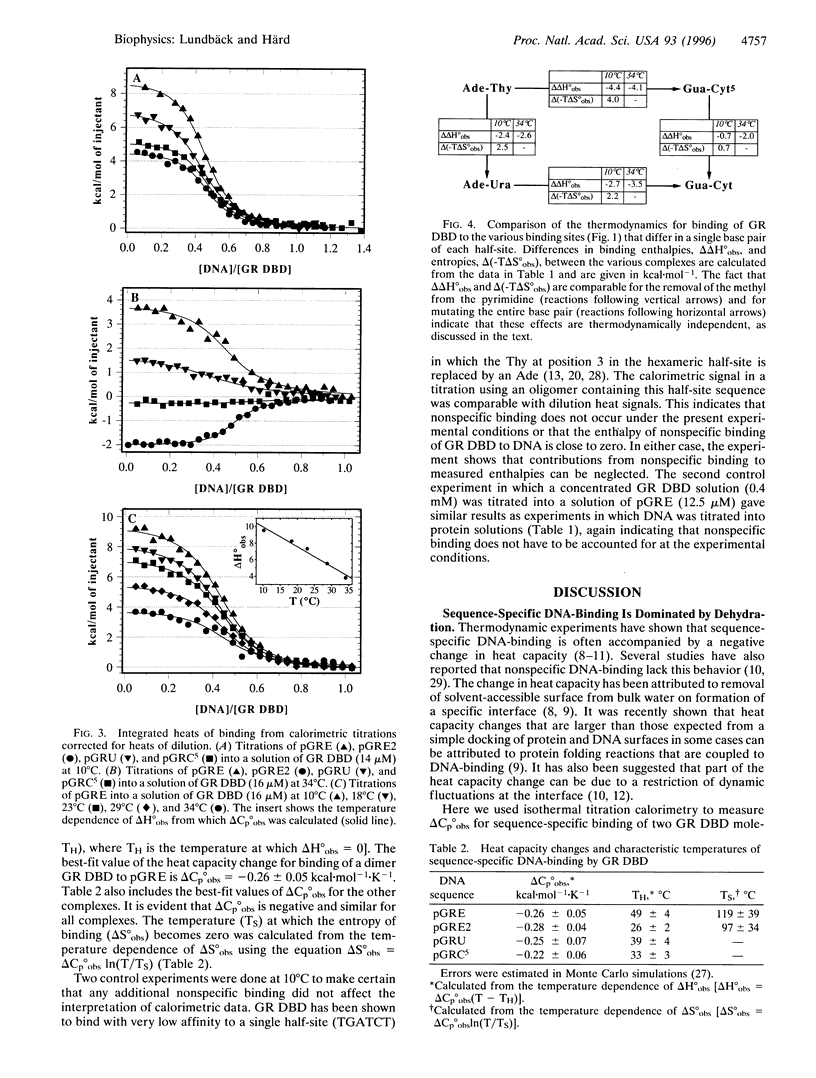
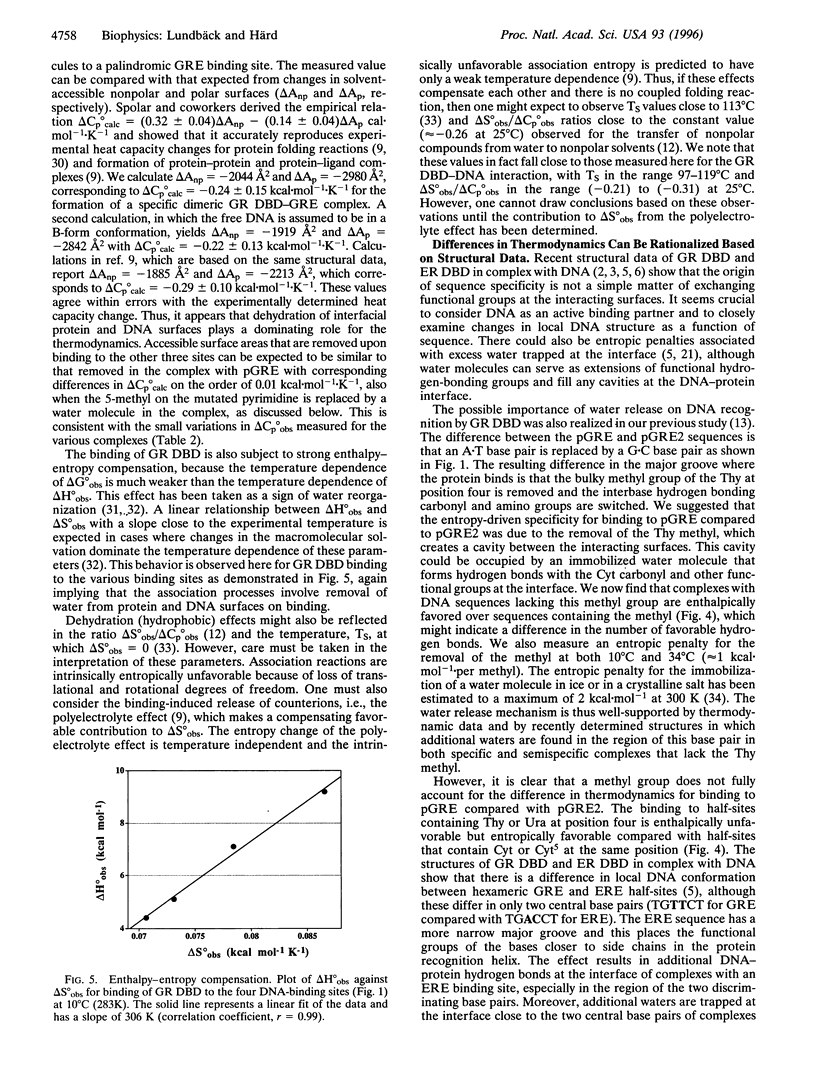
Abstract
Fluorescence spectroscopy and isothermal titration calorimetry were used to study the thermodynamics of binding of the glucocorticoid receptor DNA-binding domain to four different, but similar, DNA-binding sites. The binding sites are two naturally occurring sites that differ in the composition of one base pair, i.e., an A-T to G-C mutation, and two sites containing chemical intermediates of these base pairs. The calorimetrically determined heat capacity change (Delta C(p)o(obs)) for glucocorticoid receptor DNA-binding domain binding agrees with that calculated for dehydration of solvent-accessible surface areas. A dominating effect of dehydration or solvent reorganization on the thermodynamics is also consistent with an observed linear relationship between observed enthalpy change (Delta Ho(obs)) and observed entropy change (Delta So(obs)) with a slope close to the experimental temperature. Comparisons with structural data allow us to rationalize individual differences between Delta Ho(obs) (and Delta So(obs)) for the four complexes. For instance, we find that the removal of a methyl group at the DNA-protein interface is enthalpically favorable but entropically unfavorable, which is consistent with a replacement by an ordered water molecule.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arbuckle N. D., Luisi B. A recipe for specificity. Nat Struct Biol. 1995 May;2(5):341–346. doi: 10.1038/nsb0595-341. [DOI] [PubMed] [Google Scholar]
- Baldwin R. L. Temperature dependence of the hydrophobic interaction in protein folding. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8069–8072. doi: 10.1073/pnas.83.21.8069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann H., Paulsen K., Kovács H., Berglund H., Wright A. P., Gustafsson J. A., Härd T. Refined solution structure of the glucocorticoid receptor DNA-binding domain. Biochemistry. 1993 Dec 14;32(49):13463–13471. doi: 10.1021/bi00212a011. [DOI] [PubMed] [Google Scholar]
- Dunitz J. D. The entropic cost of bound water in crystals and biomolecules. Science. 1994 Apr 29;264(5159):670–670. doi: 10.1126/science.264.5159.670. [DOI] [PubMed] [Google Scholar]
- Eriksson M. A., Härd T., Nilsson L. Molecular dynamics simulations of the glucocorticoid receptor DNA-binding domain in complex with DNA and free in solution. Biophys J. 1995 Feb;68(2):402–426. doi: 10.1016/S0006-3495(95)80203-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foguel D., Silva J. L. Cold denaturation of a repressor-operator complex: the role of entropy in protein-DNA recognition. Proc Natl Acad Sci U S A. 1994 Aug 16;91(17):8244–8247. doi: 10.1073/pnas.91.17.8244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried M., Crothers D. M. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981 Dec 11;9(23):6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirth D. T., Sigler P. B. The basis for half-site specificity explored through a non-cognate steroid receptor-DNA complex. Nat Struct Biol. 1995 May;2(5):386–394. doi: 10.1038/nsb0595-386. [DOI] [PubMed] [Google Scholar]
- Ha J. H., Spolar R. S., Record M. T., Jr Role of the hydrophobic effect in stability of site-specific protein-DNA complexes. J Mol Biol. 1989 Oct 20;209(4):801–816. doi: 10.1016/0022-2836(89)90608-6. [DOI] [PubMed] [Google Scholar]
- Hyre D. E., Spicer L. D. Thermodynamic evaluation of binding interactions in the methionine repressor system of Escherichia coli using isothermal titration calorimetry. Biochemistry. 1995 Mar 14;34(10):3212–3221. doi: 10.1021/bi00010a010. [DOI] [PubMed] [Google Scholar]
- Härd T., Dahlman K., Carlstedt-Duke J., Gustafsson J. A., Rigler R. Cooperativity and specificity in the interactions between DNA and the glucocorticoid receptor DNA-binding domain. Biochemistry. 1990 Jun 5;29(22):5358–5364. doi: 10.1021/bi00474a022. [DOI] [PubMed] [Google Scholar]
- Härd T., Kellenbach E., Boelens R., Maler B. A., Dahlman K., Freedman L. P., Carlstedt-Duke J., Yamamoto K. R., Gustafsson J. A., Kaptein R. Solution structure of the glucocorticoid receptor DNA-binding domain. Science. 1990 Jul 13;249(4965):157–160. doi: 10.1126/science.2115209. [DOI] [PubMed] [Google Scholar]
- Ladbury J. E., Wright J. G., Sturtevant J. M., Sigler P. B. A thermodynamic study of the trp repressor-operator interaction. J Mol Biol. 1994 May 20;238(5):669–681. doi: 10.1006/jmbi.1994.1328. [DOI] [PubMed] [Google Scholar]
- Lee B., Richards F. M. The interpretation of protein structures: estimation of static accessibility. J Mol Biol. 1971 Feb 14;55(3):379–400. doi: 10.1016/0022-2836(71)90324-x. [DOI] [PubMed] [Google Scholar]
- Luisi B. F., Xu W. X., Otwinowski Z., Freedman L. P., Yamamoto K. R., Sigler P. B. Crystallographic analysis of the interaction of the glucocorticoid receptor with DNA. Nature. 1991 Aug 8;352(6335):497–505. doi: 10.1038/352497a0. [DOI] [PubMed] [Google Scholar]
- Lumb K. J., Kim P. S. Measurement of interhelical electrostatic interactions in the GCN4 leucine zipper. Science. 1995 Apr 21;268(5209):436–439. doi: 10.1126/science.7716550. [DOI] [PubMed] [Google Scholar]
- Lumry R., Rajender S. Enthalpy-entropy compensation phenomena in water solutions of proteins and small molecules: a ubiquitous property of water. Biopolymers. 1970;9(10):1125–1227. doi: 10.1002/bip.1970.360091002. [DOI] [PubMed] [Google Scholar]
- Lundbäck T., Cairns C., Gustafsson J. A., Carlstedt-Duke J., Härd T. Thermodynamics of the glucocorticoid receptor-DNA interaction: binding of wild-type GR DBD to different response elements. Biochemistry. 1993 May 18;32(19):5074–5082. doi: 10.1021/bi00070a015. [DOI] [PubMed] [Google Scholar]
- Lundbäck T., Zilliacus J., Gustafsson J. A., Carlstedt-Duke J., Härd T. Thermodynamics of sequence-specific glucocorticoid receptor-DNA interactions. Biochemistry. 1994 May 17;33(19):5955–5965. doi: 10.1021/bi00185a037. [DOI] [PubMed] [Google Scholar]
- MAHLER H. R., KLINE B., MEHROTRA B. D. SOME OBSERVATIONS ON THE HYPOCHROMISM OF DNA. J Mol Biol. 1964 Sep;9:801–811. doi: 10.1016/s0022-2836(64)80186-8. [DOI] [PubMed] [Google Scholar]
- Marky L. A., Breslauer K. J. Origins of netropsin binding affinity and specificity: correlations of thermodynamic and structural data. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4359–4363. doi: 10.1073/pnas.84.13.4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pabo C. O., Sauer R. T. Transcription factors: structural families and principles of DNA recognition. Annu Rev Biochem. 1992;61:1053–1095. doi: 10.1146/annurev.bi.61.070192.005201. [DOI] [PubMed] [Google Scholar]
- Richmond T. J. Solvent accessible surface area and excluded volume in proteins. Analytical equations for overlapping spheres and implications for the hydrophobic effect. J Mol Biol. 1984 Sep 5;178(1):63–89. doi: 10.1016/0022-2836(84)90231-6. [DOI] [PubMed] [Google Scholar]
- Schwabe J. W., Chapman L., Finch J. T., Rhodes D. The crystal structure of the estrogen receptor DNA-binding domain bound to DNA: how receptors discriminate between their response elements. Cell. 1993 Nov 5;75(3):567–578. doi: 10.1016/0092-8674(93)90390-c. [DOI] [PubMed] [Google Scholar]
- Schwabe J. W., Chapman L., Rhodes D. The oestrogen receptor recognizes an imperfectly palindromic response element through an alternative side-chain conformation. Structure. 1995 Feb 15;3(2):201–213. doi: 10.1016/s0969-2126(01)00150-2. [DOI] [PubMed] [Google Scholar]
- Schwabe J. W., Neuhaus D., Rhodes D. Solution structure of the DNA-binding domain of the oestrogen receptor. Nature. 1990 Nov 29;348(6300):458–461. doi: 10.1038/348458a0. [DOI] [PubMed] [Google Scholar]
- Spolar R. S., Livingstone J. R., Record M. T., Jr Use of liquid hydrocarbon and amide transfer data to estimate contributions to thermodynamic functions of protein folding from the removal of nonpolar and polar surface from water. Biochemistry. 1992 Apr 28;31(16):3947–3955. doi: 10.1021/bi00131a009. [DOI] [PubMed] [Google Scholar]
- Spolar R. S., Record M. T., Jr Coupling of local folding to site-specific binding of proteins to DNA. Science. 1994 Feb 11;263(5148):777–784. doi: 10.1126/science.8303294. [DOI] [PubMed] [Google Scholar]
- Sturtevant J. M. Heat capacity and entropy changes in processes involving proteins. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2236–2240. doi: 10.1073/pnas.74.6.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y., Ross P. D., Mudd C. P. Thermodynamics of Cro protein-DNA interactions. Proc Natl Acad Sci U S A. 1992 Sep 1;89(17):8180–8184. doi: 10.1073/pnas.89.17.8180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilliacus J., Wright A. P., Norinder U., Gustafsson J. A., Carlstedt-Duke J. Determinants for DNA-binding site recognition by the glucocorticoid receptor. J Biol Chem. 1992 Dec 15;267(35):24941–24947. [PubMed] [Google Scholar]


