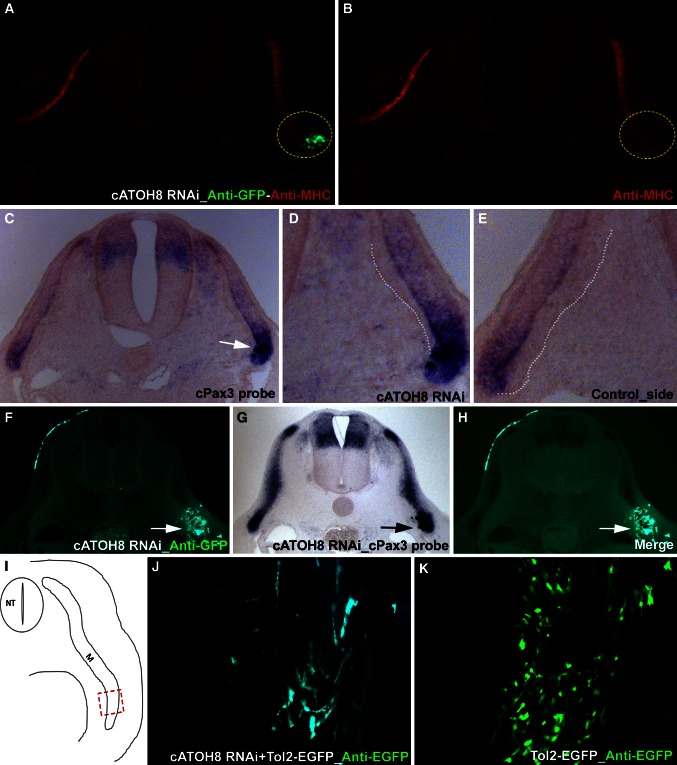
Fig. 3.
Silencing ATOH8 in the somites affects muscle differentiation and cellular arrangement. The embryos electroporated with ATOH8-specific shRNA-EGFP constructs at the lateral somites were reincubated and processed for immunohistochemistry (myosin heavy chain—MHC). The targeted region is indicated by the EGFP expression (immunohistochemistry) (a). ATOH8 silencing leads to the downregulation of MHC (dotted circle in b) at the targeted site (dotted circle in a). A section through the same embryo presented before in Fig. 2 j–m shows a clear upregulation of Pax3 at the ATOH8 shRNA electroporated region (c). The enlarged view of c shows defective hypaxial myotome formation after ATOH8 knockdown (white dotted line in d). The normal myotome formation is shown on the control side (white dotted line in e). Anti-EGFP immunohistochemistry on the cross-sections of ATOH8 shRNA electroporated and Pax3 stained (in situ hybridization) embryo shows the co-localization of Pax3 upregulation and EGFP expression, which indicates ATOH8 shRNA (white arrows in f, h, black arrow in g). ATOH8 RNAi (visualized here by co-electroporation of constructs in combination with the Tol2-EGFP expression system) in the hypaxial dermomyotome resulted in a remarkable distortion of hypaxial myotome development and caused patterning defects in the myofibers 4 days after electroporation (j). Electroporation of the Tol2 stable expression system alone did not show any distortion of myotome formation (k). Red dashed rectangle (i) indicates the hypaxial region in the embryo shown in j and k. NT neural tube, M myotome