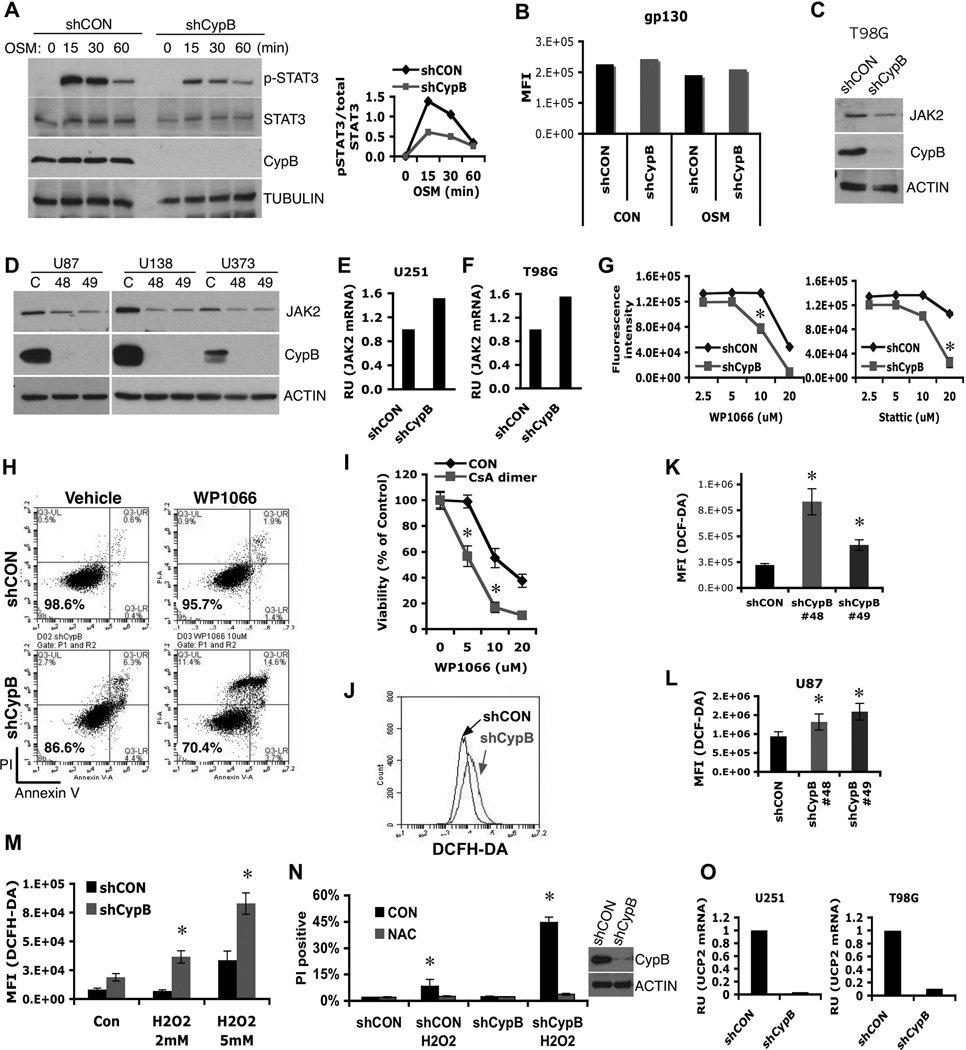
Figure 2. Cyclophilin B controls Jak2-Stat3 signals and ROS generation.
(A) Control or CypB-knockdown U251 cells were treated with Oncostatin M (OSM) for the indicated times and analyzed by westernblotting with the indicated antibodies (left). The results of phospho-Stat3 (pStat3) and total Stat3 westernblots were quantified using ImageJ. Relative intensity values of pStat3 to total Stat3 were plotted (right). (B) Normal cell surface expression of gp130 in CypB-depleted U251 cells, as measured by flow cytometry. (C and D) CypB depletion reduced Jak2 protein abundance in T98G, U87, U138, and U373 GBM cells. (E and F) Knockdown of CypB did not reduce Jak2 mRNA levels in U251 and T98G cells, measured by real-time PCR. (G and H) Decreased cell viability in CypB-depleted U251 cells in response to treatment with Stat3 inhibitors. Control or CypB-depleted U251 cells were treated with the indicated doses of Stat3 inhibitor, WP1066 (left) or Stattic (right) for 48h (G). U251 cells were treated with WP1066 (10µM) for 24h and cell viability was quantified by annexin-V/PI staining (H). (I) Increased cell killing effects with the indicated compounds for 24hr. Cell viability was observed by AlamarBlue assay. (J–L) Knockdown of CypB increased ROS generation in T98G (J), U251 (K), and U87 cells (L). Cellular ROS was measured using flow cytometry after staining with DCFH-DA (0.5mM for 30min). (M) CypB-depleted T98G cells had higher ROS production in response to H2O2 treatment. Cells were treated with H2O2 for 2hr and cellular ROS was measured by DCFH-DA staining. (N) CypB-depleted T98G cells showed increased sensitivity to oxidative stress-induced cell death. Cells were pretreated with 5mM N-acetyl cysteine (NAC) or vehicle, and subsequently with H2O2 for 24h. Cellular viability was measured by PI stain exclusion. (O) CypB silencing decreased UCP2 mRNA levels in U251 (left) or T98G (right) cells. Errorbars show ±SD. *p<0.05.