Abstract
Obstructive nephropathy leads to chronic kidney disease, characterized by a progressive epithelial-to-mesenchymal cell transition (EMT)-driven interstitial fibrosis. To identify the mechanisms causing EMT, we used the mouse model of unilateral ureteral obstruction and found a rapid and significant increase in serum- and glucocorticoid-regulated kinase-1 (SGK1) expression in the kidneys with an obstructed ureter. Knockout of SGK1 significantly suppressed obstruction-induced EMT, kidney fibrosis, increased glycogen synthase kinase-3β activity, and decreased accumulation of the transcriptional repressor Snail. This caused a reduced expression of the mesenchymal marker α-smooth muscle actin, and collagen deposition in this model. In cultured kidney epithelial cells, mechanical stretch or treatment with transforming growth factor-β not only stimulated the transcription of SGK1, but also stimulated EMT in an SGK1-dependent manner. Activated SGK1 stimulated Snail accumulation and downregulation of the epithelial marker E-cadherin. Hence, our study shows that SGK1 is involved in mediating fibrosis associated with obstructive nephropathy.
Keywords: epithelial-to-mesenchymal transition, fibrosis, glycogen synthase kinase, obstructive nephropathy, SGK1, Snail
Obstructive nephropathy due to congenital abnormalities is a primary cause of end-stage renal disease in children;1 its incidence increases when patients are above 60–65 years. Understanding the mechanisms underlying interstitial fibrosis and tubular atrophy that occurs with obstruction can lead to methods for preventing the loss of kidney function.2,3 Unilateral ureteral obstruction (UUO) in rats or mice is a model of obstructive nephropathy with tubulointerstitial fibrosis, cellular infiltration, tubular proliferation, and apoptosis.4 Epithelial-to-mesenchymal cell transition (EMT) and increased extracellular matrix (ECM) deposition contribute to UUO-induced fibrosis. Several mechanisms have been identified contributing to the fibrosing effect of obstructive nephropathy, such as mechanical stretch,5,6 angiotensin II (renin–angiotensin system),7,8 and transforming growth factor-β (TGF-β).9 Mechanical stretch is an important factor involved in EMT, as others have reported that mechanical stretch induces TGF-β expression.5,6 Blocking the TGF-β signaling pathway suppresses mechanical stretch-induced EMT in kidney epithelial cells.
Epithelial cells form layers that have apical–basolateral polarization, clustering E-cadherin-formed adhesions between epithelial cells.10 Epithelial cells can be transdifferentiated into mesenchymal cells by EMT, which is paralleled by loss of E-cadherin expression.11 Mesenchymal cells have a spindle-shaped, fibroblast-like morphology expressing specific markers, such as smooth muscle α-actin (α-SMA), fibronectin, and vimentin.11 E-cadherin transcription is downregulated by the transcriptional factor Snail;12,13 Snail expression is upregulated by TGF-β,14,15 insulin-like growth factor 1,16 or mechanical stretch.6 Moreover, Snail activity is regulated by glycogen synthase kinase-3β (GSK-3β), because it phosphorylates Snail and then stimulates its degradation.17 Phosphatidylinositol 3-kinase (PI3K), integrin-linked kinase (ILK), and Wnt signaling pathways reportedly promote EMT in a model of UUO.18–22 However, the intracellular signaling accounting for kidney fibrosis in UUO is incompletely understood.
Serum- and glucocorticoid-regulated kinase 1 (SGK1) is transcriptionally regulated by serum and glucocorticoids.23,24 It is a member of the AGC (cAMP-dependent protein kinase A, cGMP-dependent kinase G, and phospholipid-dependent protein kinase C) family of serine or theronine protein kinases and a downstream signaling element in the PI3K pathway. Recent studies have implicated a critical role of SGK1 in the regulation of cell survival, proliferation, and differentiation. SGK1 further regulates a wide variety of channels and transporters, including the epithelial sodium channel.25 Ample evidence points to a critical role of SGK1 in the pathophysiology of fibrosis.25–29 SGK1 has been shown to mediate the epidermal growth factor-induced expression of fibronectin in kidney proximal cells.30 Moreover, lack of SGK1 knockout (KO) prevents the stimulation of connective tissue growth factor expression and cardiac fibrosis following mineralocorticoid excess.31 In humans, an SGK1 gene variant is associated with enhanced blood pressure,32,33 increased body weight34, and diabetes.35
The purpose of the present study was to identify the role of SGK1 in the intracellular signaling underlying renal fibrosis after unilateral ureteral obstruction.
RESULTS
UUO-induced fibrosis is regulated by SGK1
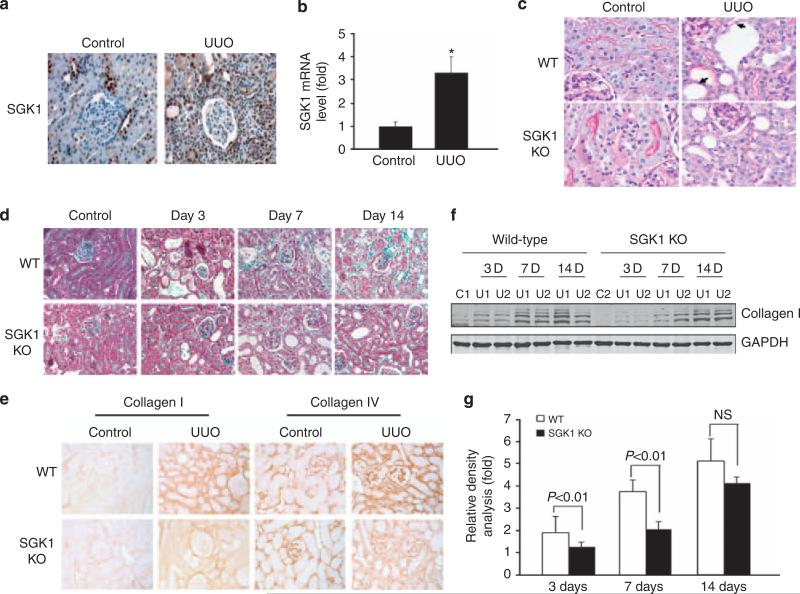
At 3 days after UUO, expression of SGK1 protein and mRNA was increased in the obstructed kidney as compared with the unobstructed, contra-lateral kidney (Figure 1a and b). As shown in Figure 1c, UUO was followed by damage of the apical brush border structures, thickening of the tubule basement membrane, and increase of cell number in the interstitium of wild-type (WT) kidneys. In contrast, kidneys from SGK1 KO mice were protected from UUO-induced injury (Figure 1c). The blue color of trichrome staining in Figure 1d indicates ECM proteins. In the kidneys of WT mice, 7 or 14 days of UUO significantly increased the ECM protein levels. The effect of UUO on ECM protein deposition was again significantly blunted in SGK1 KO mice (Figure 1d). Specifically, UUO in WT mice increased the levels of collagens I and IV, primarily in the interstitial and peritubular areas (Figure 1e). The effect of UUO on collagen deposition was reduced by 40% (collagen-I) and 57% (collagen-IV) in SGK1 KO mice (Figure 1e). The reduction of collagen abundance was confirmed by western blotting (Figure 1e–g). The decrease of collagen deposition in SGK1 KO mice was paralleled by a similar suppression of fibronectin expression (data not shown).
Figure 1. Unilateral ureteral obstruction (UUO)-induced kidney fibrosis was suppressed in serum- and glucocorticoid-regulated kinase 1 (SGK1) knockout (KO) mice.
(a) SGK1 expression in vivo was increased at 3 days after UUO. (b) Real time reverse transcriptase-PCR analysis of UUO-induced SGK1 expression. (c) Kidney sections from wild-type (WT) and SGK1 KO mice with or without UUO for 7 days were stained by periodic acid-Schiff. The arrows point to damaged tubular cells and basement membrane thickening. (d) Sections from control and obstructed kidneys from WT and SGK1 KO mice were stained with Masson's-modified trichrome. The blue color shows extracellular collagen deposition. (e) SGK1 KO inhibited UUO-induced collagen accumulation that was detected by immunostaining with anti-collagen I or anti-collagen IV antibodies and processed with 3,3′-diaminobenzidine (DAB) (brown color). (f) SGK1 KO suppressed UUO-induced collagen I expression. Cell lysates were prepared from control and UUO-treated kidney cortex in WT and SGK1 KO mice after UUO at day 0, 3, 7, and 14. Collagen I expression level was detected by western blot. GAPDH was used as internal loading control. (g) Density analysis of (e). Data represent three repeated experiments. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
UUO-induced EMT is suppressed in SGK1 KO mice
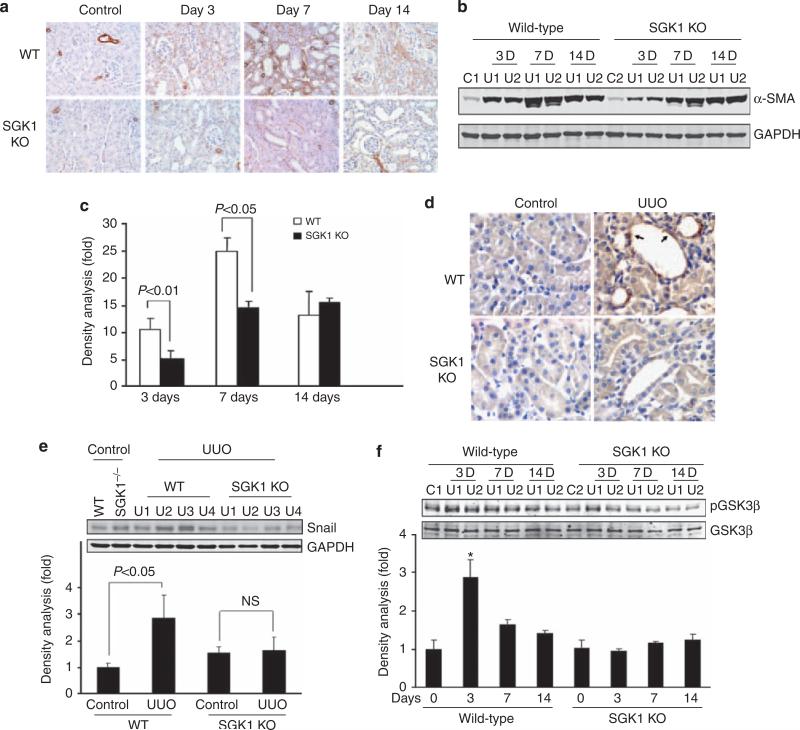
As mesenchymal cells were derived from epithelial cells expressing α-SMA, we measured α-SMA expression at different time points after UUO using immunohistochemical staining (Figure 2a) and western blotting (Figure 2b). After 3 days of UUO, α-SMA was significantly increased and continued to increase further for the next 7 days as compared with the results in the contra-lateral control kidney (Figure 2a–c). In contrast, α-SMA expression in the obstructed kidneys of SGK1 KO mice was markedly attenuated, as compared with kidneys from obstructed WT mice (Figure 2a–c, at day 3). However, the protective effect was dissipated at 14 days (Figure 2a–c).
Figure 2. Unilateral ureteral obstruction (UUO)-induced epithelial–mesenchymal transition (EMT) was suppressed by serum- and glucocorticoid-regulated kinase 1 (SGK1) knockout (KO) in vivo.
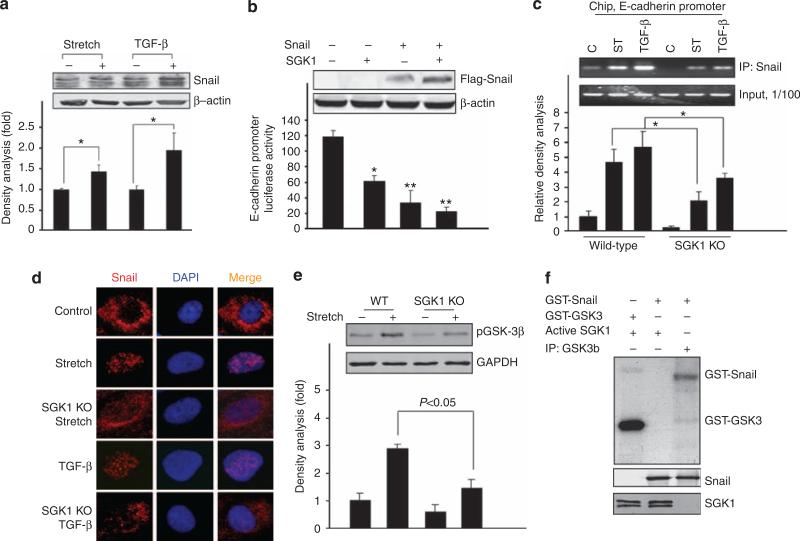
(a, b) UUO-induced α-smooth muscle actin (SMA) expression was attenuated by SGK1 KO. UUO surgery was carried out in wild-type (WT) and SGK1 KO mice; α-SMA was determined by immunohistochemistry (a) and western blot analysis (b). GAPDH was used as loading control. (c) Density analysis of panel (b). (d) SGK1 KO attenuates UUO-induced Snail expression. Kidney sections were prepared from control and UUO-treated (3 days) kidneys from WT and SGK1 KO mice. Immunohistochemistry was carried out with anti-Snail antibody; the positive cells are shown in brown color. The arrows point to the Snail-positive cells in dilated lumen in kidney tubule. (e, f) Western blot analysis of Snail expression (e) and glycogen synthase kinase-3β (GSK-3β) phosphorylation in UUO-treated WT and SGK1 KO mice. The bar graph shows the density analysis of the western blots. NS, non-significant. *P<0.05 compared with control.
To explore how SGK1 mediates EMT, we assessed the expression of Snail, a transcriptional repressor of E-cadherin, in UUO kidneys. As shown in Figure 2d (indicated by arrow), UUO significantly increased Snail levels in kidneys from WT mice but not in those from SGK1 KO mice (Figure 2d and e). UUO treatment significantly increased GSK-3β phosphorylation in kidneys from WT mice, an effect not observed in kidneys from SGK1 KO mice (Figure 2f).
Mechanical stretch or TGF-β stimulates SGK1 expression and EMT in cultured kidney tubule cells
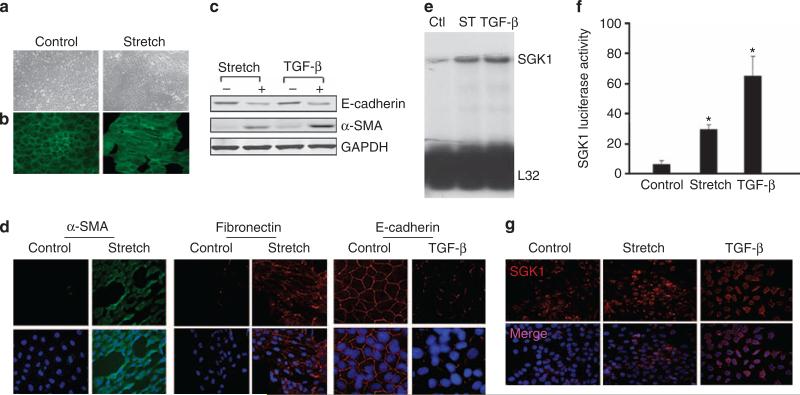
In the UUO model, there are many compounding factors, such as infiltration of inflammatory cells, production of cytokines, growth factors, and accumulation of metabolites. It was reported that stretch or TGF-β, two of the major underlying factors, contributes to kidney fibrosis in UUO. To establish a cell model for UUO and to identify the mechanisms for EMT, we examined how factors that are known to be effective in UUO do regulate SGK1 expression in cultured kidney tubule cells. In cultured kidney cells from WT mice, there was a characteristic cuboidal appearance with F-actin located around the cell membrane. Tubule cells and F-actin were in parallel along the direction of the force induced by mechanical stretch (Figure 3a and b). Treatment of tubule cells with stretch or TGF-β significantly decreased the level of the epithelial marker, E-cadherin, and increased the level of mesenchymal cell markers, α-SMA and fibronectin (Figure 3c and d). Stretch or TGF-β also induced SGK1 mRNA expression (Figure 3e) and SGK1 transcriptional activity (Figure 3f). Furthermore, mechanical stretch or TGF-β treatment increased SGK1 protein expression (Figure 3g).
Figure 3. Both mechanical stretch and transforming growth factor (TGF)-β induced epithelial–mesenchymal transition (EMT) and serum- and glucocorticoid-regulated kinase 1 (SGK1) expression.
(a, b) Mechanical stretch changed cell morphology and cytoskeleton polarity in kidney epithelial cells. Cells were seeded into silicone elastomer-bottomed six-well plates, and the cell morphology (a) and F-actin (b) were photographed after 48-h stretch. (c, d) Both mechanical stretch and TGF-β downregulate E-cadherin levels. Kidney epithelial cells were treated with 3 ng/ml TGF-β or mechanical stretch, the protein levels of E-cadherin, α-smooth muscle actin (SMA), and fibronectin were detected by western blot analysis (c) and by immunofluorescence staining (d). (e) Mechanical stretch (ST) or TGF-β induces SGK1 mRNA level. Ribonuclease protection assay was carried out to detect the SGK1 mRNA level. L32 was used as a loading control. (f) Mechanical stretch or TGF-β can increase SGK1 promoter transcription activity. Cells were transfected with pSGK1-Luc and renilla luciferase plasmid for 24 h, and were treated with mechanical stretch or TGF-β. The luciferase activity was determined after 24 h and normalized with renilla luciferase. The results represent three independent experiments. *P<0.05 compared with control. (g) Mechanical stretch or TGF-β caused SGK1 expression. Ctl, control.
SGK1 is a downstream signal that mediates mechanical stretch- and TGF-β-induced EMT
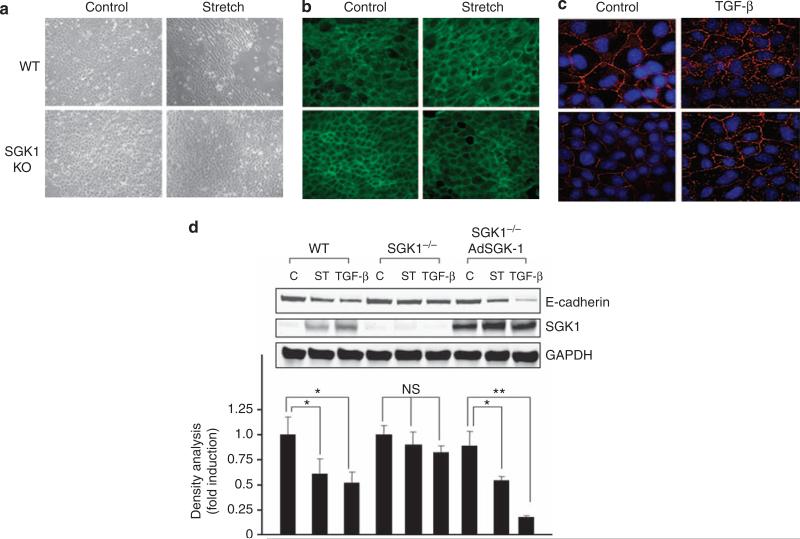
To define the importance of SGK1 in UUO-induced fibrosis, we isolated tubule cells from WT and SGK1 KO mice and subjected them to TGF-β or stretch. Stretch induced striking changes in cell morphology and cytoskeletal reorganization (Figure 4). In cells from SGK1 KO mice, less F-actin redistributed in the direction of mechanical force when compared with the results in tubule cells from WT mice (Figure 4a and b). According to the immunofluorescent staining result, TGF-β downregulated E-cadherin expression, an effect blunted by 54% in SGK1 KO cells (Figure 4c). In contrast to E-cadherin levels in wild cells, the protein level of E-cadherin remained high in SGK1 KO cells despite mechanical stretch or TGF-β treatment (Figure 4d). Transfection of SGK1 into SGK1 KO cells using an adenoviral construct rescued both the mechanical stretch- and the TGF-β-mediated decrease in E-cadherin protein level (Figure 4d).
Figure 4. Serum- and glucocorticoid-regulated kinase 1 (SGK1) knockout (KO) suppressed mechanical stretch- or transforming growth factor (TGF)-β-induced epithelial–mesenchymal transition (EMT) in vitro.
(a, b) SGK1 KO inhibited mechanical stretch-induced cell morphology and cytoskeleton rearrangement. Kidney tubular cells from wild-type (WT) and SGK1 KO mice were treated with mechanical stretch for 24 h, and the morphology (a) and F-actin staining (b) were detected. (c) TGF-β-induced E-cadherin downregulation was inhibited by SGK1 KO. (d) Reexpression of SGK1 in SGK1 KO cells rescued the mechanical stretch- or TGF-β-caused E-cadherin downregulation. The expression of SGK1 in SGK1 KO cells was achieved by infection of SGK1 expression adenovirus. The lower panel shows the densitometry analysis. The data represent three independent experiments. *P<0.05 compared with control. **P<0.01 compared with control.
SGK1 downregulates E-cadherin through Snail
Snail is a transcriptional suppressor that decreases E-cadherin expression. Treatment of tubule cells with stretch or TGF-β increased Snail expression (Figure 5a). To study whether SGK1 regulates Snail activity and E-cadherin transcription, kidney tubule cells were co-transfected with an E-cadherin promoter–reporter construct with SGK1 or Snail expression plasmids. As shown in Figure 5b, overexpression of either Snail or SGK1 significantly decreased E-cadherin promoter activity in tubular cells. Combined expression of Snail and SGK1 again significantly decreased the luciferase level, consistent with a pathway from SGK1 to Snail leading to suppression of E-cadherin. This conclusion was further supported by the observation that overexpression of SGK1 stabilized the Snail protein (inset in Figure 5b).
Figure 5. Mechanical stretch- and transforming growth factor (TGF)-β-stimulated Snail expression and activation was blocked by serum- and glucocorticoid-regulated kinase 1 (SGK1) knockout (KO).
(a) Mechanical stretch and TGF-β induced Snail expression. (b) Snail and SGK1 inhibited E-cadherin promoter activity. Tubular cells were transfected with pE-cadherin-luc plasmid alone or co-transfected with pcDNA-flag-Snail or constitutive active pcDNA-SGK1 (256D); the luciferase activity was assayed after 24 h. The inset shows western blot. Data represent three independent experiments. *P<0.05 compared with control. **P<0.01 compared with control. (c) SGK1 KO suppressed Snail DNA-binding activity. Wild-type (WT) and SGK1 KO kidney tubular cells were subjected to mechanical stretch or TGF-β; the chromatin immunoprecipitation assay was carried out following the method described in Materials and Methods. (d) SGK1 KO blocked mechanical stretch- or TGF-β-induced Snail nuclear translocation. Kidney tubular cells from WT and SGK1 KO mice were transfected with pCMV-flag-Snail expression plasmid for 24 h and then subjected to mechanical stretch or TGF-β for 8 h. The immunofluorescence staining using anti-flag antibody was carried out. The photographs were taken using a deconvolution microscope. (e) SGK1 KO inhibited mechanical stretch-induced glycogen synthase kinase-3β (GSK-3β) phosphorylation. (f) SGK1 cannot directly phosphorylate Snail. Purified recombinant GST-Snail was incubated with either purified activated recombinant SGK1 or GSK-3β in 30 μl of kinase reaction buffer containing γ-32P-ATP; the labeled signals were detected through radioactivity exposure. The GST-GSK-3β was used as positive control for SGK1 kinase activity.
To further explore how SGK1 regulates E-cadherin expression by Snail, we carried out a chromatin immunoprecipitation assay. Mechanical stretch or TGF-β stimulated the Snail-binding capacity to the E-cadherin promoter in WT cells, an effect significantly blunted in tubule cells isolated from SGK1 KO mice (Figure 5c).
In primary cultures of tubule cells derived from WT mice, mechanical stretch or TGF-β also induced translocation of Snail into nuclei. This nuclear translocation did not occur in cells isolated from SGK1 KO mice (Figure 5d).
Further experiments were performed to elucidate the mechanism accounting for the SGK1-dependent nuclear translocation of Snail. As shown in Figure 5e, lack of SGK1 blocked stretch-induced phosphorylation of GSK-3β. Incubation of recombinant GSK-3β with glutathion S-transferase (GST)-Snail resulted in Snail phosphorylation. Incubation of recombinant SGK1 with GST-Snail did not result in Snail phosphorylation, indicating that SGK1 increased Snail phosphorylation by GSK-3β (Figure 5f).
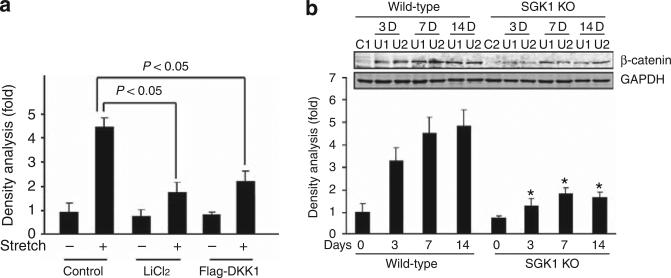
SGK1 downregulates β-catenin expression in UUO-treated kidney. DKK1 functions as an inhibitor of the β-catenin signaling pathway by binding with Wnts. Overexpression of DKK1 or inhibition of GSK-3 in kidney tubule cells significantly inhibited mechanical stretch-stimulated Snail expression (Figure 6a). In SGK1 KO mice, the UUO-induced β-catenin was suppressed compared with the β-catenin in WT mice (Figure 6b).
Figure 6. Serum- and glucocorticoid-regulated kinase 1 (SGK1) knockout (KO) inhibits unilateral ureteral obstruction (UUO)-induced β-catenin.
(a) Mice kidney tubule cells were transfected with control vector or flag-DKK1 for 24 h or treated with GSK3 inhibitor, lithium chloride, before being subjected to mechanical stretch. The Snail mRNA levels were detected by real-time reverse transcriptase-PCR. (b) SGK1 KO suppressed UUO-induced β-catenin expression. Cell lysates were prepared from control and UUO-treated kidney cortex in wild-type and SGK1 KO mice after UUO at day 0, 3, 7, and 14. β-Catenin expression level was detected by western blot. GAPDH was used as internal loading control. Data represent three repeated experiments. *P<0.05.
DISCUSSION
Several studies indicate that obstructive nephropathy causes transition of tubular epithelial to mesenchymal cells and fibrosis in the kidney, but the underlying mechanism has remained largely elusive. In this study, we have discovered that SGK1 is a critical signaling molecule regulating the transition of tubular epithelial to mesenchymal cells and fibrosis during ureteral obstruction. The obstruction triggers a stretch-sensitive, TGF-β-inducible SGK1 expression, and a signaling pathway that upregulates Snail transcriptional factor, leading to downregulation of E-cadherin.
Multiple signaling pathways reportedly promote EMT.9,21,36–38 Liu et al.37 reported that the ILK signaling pathway has a critical role in mediating tubular EMT and renal interstitial fibrosis, and that PINCH-1 is involved in this process. PINCH is an adaptor protein that binds to ILK and regulates cytoskeleton structure. Overexpression of PINCH-1 decreases E-cadherin and ZO-1 (a tight-junction molecule in epithelial cells) levels leading to EMT.39 Evidence for the involvement of the PI3K pathway in the regulation of EMT has been provided by studies on human cancer.21 However, the signaling mediating the stimulation of EMT by PI3K or ILK remained elusive. ILK has been suggested to operate as a PDK2, which can activate cAMP-dependent protein kinase A, cGMP-dependent kinase G, and phospholipid-dependent protein kinase C family protein kinases, including Akt and SGK1.40
In this study we show that SGK1 is a critical mediator for EMT. SGK1 is a serine/threonine protein kinase with 45–50% sequence identity in its catalytic domain with protein kinase B (Akt) and protein kinase C. It is activated by PDK1 (3-phosphoinositide-dependent kinase)-dependent phosphorylation of Thr256 and, therefore, is modulated by PI3K.23,41,42 We found that SGK1 is significantly increased in an animal model of UUO (Figure 1). Stimuli such as TGF-β or mechanical stretch stimulate SGK1 expression (promoter transcriptional activity, RNA, and protein) in kidney tubule cells (Figure 3). These findings are important as SGK1 is a critical downstream signal of PI3K and ILK that mediate EMT under obstructive nephropathy. Importantly, KO of SGK1 suppresses UUO-induced EMT and fibrosis (Figures 1 and 2). Our results point to SGK1 as a critical factor regulating UUO-induced EMT (Figure 1). The involvement of SGK1 was shown in vitro and in response to UUO in vivo. In this model of UUO, SGK1 promoted EMT. When SGK1 was knocked out, the stimulation of EMT by UUO was suppressed (Figure 1). It should be pointed that the protective effects of the SGK1 KO were attenuated at day 7 but not at day 14 after UUO (Figures 1f and g). The potential mechanism could be that other factors (for example, inflammatory cell infiltration, cytokine (TGF-β secretion)) are activated beyond day 7 to induce UUO-induced EMT and fibrosis.
Several reports suggest that SGK1 could be involved in fibrosis. For example, transcription of SGK1 parallels the course of diabetic nephropathy,28,43 glomerulonephritis,44 pulmonary fibrosis,45 vascular remodeling,27 or cardiac fibrosis.31 There is also evidence that SGK1 promotes ECM protein accumulation in the heart, possibly through the connective tissue growth factor. Indeed, expression of the connective tissue growth factor was linked to SGK1.31 SGK1 also controls the expression of fibronectin in endothelial cells46 and tissue factor during vascular remodeling27 or lung fibrosis.45 According to our observations, SGK1-dependent EMT may explain that pro-fibrotic stimuli such as HGF, TGF-β, and integrin47 induce fibrosis by activation of SGK1.
The present observations further provide insight into the SGK1-dependent mechanisms leading to EMT. UUO increases Snail accumulation of kidney tubule cells (Figure 2). This observation is consistent with reports that UUO induces the expression of Snail in renal tubular epithelial cells.6,16 According to our observations, the influence of UUO on Snail abundance was at least partially mediated by SGK1, as the accumulation of Snail after UUO was significantly blocked in kidneys of SGK1 KO mice. Similarly, the effect of mechanical stretch or TGF-β on Snail abundance in cultured kidney tubular epithelial cells was sensitive to the presence of SGK1 (Figure 2). SGK1 increases Snail stability and expression SGK1 in tubule cells leads to Snail accumulation (Figure 5). The effect of SGK1 on Snail is mediated by GSK-3β, as increased GSK-3β activity causes Snail phosphorylation and degradation. As SGK1 decreases GSK-3β kinase activity, it abrogates the effect of GSK-3β on Snail phosphorylation and degradation. According to our observations, SGK1 does not directly phosphorylate Snail, but is rather effective owing to its influence on GSK-3β (Figure 5). SGK1 reduces GSK-3β-induced Snail phosphorylation and the subsequent Snail degradation.17,38 The Snail transcription factor represses E-cadherin expression and promotes EMT.48 Mechanical stretch or TGF-β fosters Snail binding to the E-cadherin promoter (Figure 5). According to our observations, UUO or the mechanical stretch of cultured cells stimulates GSK-3β phosphorylation in WT mice or cells derived from WT mice, but not in SGK1 KO mice or cells derived from SGK1 KO mice (Figures 2f and 5f). GSK-3β functions as a downstream signal of the PI3K and Wnt pathways, which are linked to the development of EMT.17,21,36 Finally, we found a critical role for the GSK-3β/β-catenin signaling pathway in mechanical stretch-induced Snail expression and for SGK1 in regulation of β-catenin. Blocking the Wnt signaling pathway by DKK-1 or GSK-3β by lithium chloride inhibits mechanical stretch-induced Snail expression (Figure 6a). Moreover, UUO induced β-catenin protein level that was suppressed in SGK1 KO mice (Figure 6b). These results are consistent with the recent finding that the Wnt/β-catenin pathway is involved in renal fibrosis.49
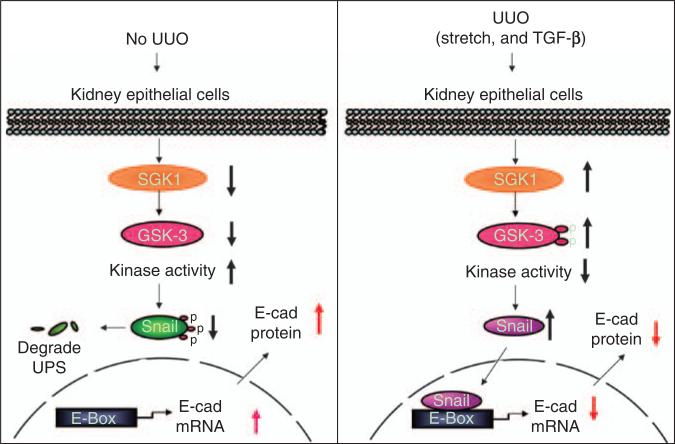
In view of our results, we propose a signaling pathway from SGK1 activation to EMT (Figure 7). In normal kidneys, SGK1 expression and hence the level of phosphorylated GSK-3β are low. Consequently, GSK-3β kinase activity is sufficient to phosphorylate Snail, leading to its degradation. In UUO, stimuli such as stretch of kidney epithelial cells or increased TGF-β stimulate SGK1 expression and activation, leading to phosphorylation of GSK-3β and decreased GSK-3β kinase activity. As a result, Snail phosphorylation and degradation are suppressed. When Snail is increased, it suppresses E-cadherin transcription, thus fostering EMT.
Figure 7. Cartoon illustrating the role of serum- and glucocorticoid-regulated kinase 1 (SGK1) in unilateral ureteral obstruction (UUO)-induced epithelial–mesenchymal transition (EMT) signaling.
The left panel shows normal condition; the expression of SGK1 and glycogen synthase kinase-3β (GSK-3β) phosphorylation level are low, which causes phosphorylation of Snail and its subsequent degradation; E-cadherin expression level is maintained. The right panel shows UUO. The SGK1 expression is high and is followed by increased GSK-3β phosphorylation and decreased kinase activity. The Snail phosphorylation and degradation are blocked. After translocation to the nucleus, Snail binds with the E-box in the E-cadherin (E-cad) promoter, which suppresses E-cadherin expression and thus causes EMT. TGF-β, transforming growth factor-β.
In summary, in a mouse model of UUO, we have shown that SGK1 is a critical factor for the development of EMT and fibrosis. KO of SGK1 suppressed pathological responses and changed Snail expression and its phosphorylation, stabilization, and localization in kidney tubular cells.
MATERIALS AND METHODS
UUO model in mice
WT and SGK1 KO mice were generated as described before.50 Six WT and SGK1 KO mice, of 20–30 g body weight, in each group were studied at 6–8 weeks of age and UUO was carried out as described.37 Sham-operated mice had their ureter exposed but not ligated. All experimental procedures were approved by the Animal Care and Use Committee of Baylor College of Medicine.
Primary culture of renal tubular epithelial cells
Minced kidneys were washed in three changes of cold phosphate-buffered saline (PBS) containing 1 mm EDTA and were digested in 0.25% trypsin solution in a shaking incubator at 37 °C for 2 h. Trypsin was neutralized with growth medium consisting of Dulbecco's modified Eagle's medium and 10% fetal bovine serum plus 100 units per ml penicillin and 0.1 mg per ml streptomycin. The suspension was triturated by pipetting and passed through a 100-μm cell strainer (Becton Dickinson Labware, Franklin Lakes, NJ, USA). The filtrate, consisting mostly of dispersed renal tubules, was plated onto culture dishes (Nalge Nunc International, Naperville, IL, USA), or onto silicone elastomer-bottomed and collagen-coated plates (Flexcell 4000, Flexcell, Hillsborough, NC, USA). The cells were incubated at 37 °C in a CO2 incubator with the media changed every 2 days. Experiments were carried out in serum-free DMEM.
Plasmids and transfection
The pCMV-flag-Snail expression plasmid and pE-cadherin promoter luciferase plasmid were gifts of Dr Zhou (Galveston, TX). The SGK1 constitutive active plasmid, pcDNA3-SGK256D, was a gift of Dr Brickley (University of Chicago, Chicago, IL). pSGK1 promoter luciferase and pSGK1 promoter cat plasmids were provided by Dr Richards (Baylor College of Medicine, Houston, TX). The Flag-DKK1 expression plasmid was kindly provided by Dr He Xi (Harvard University, Boston, MA, USA). Adenovirus expressing human SGK1 was generated by inserting SGK1 cDNA into the pAd-Tracker-CMV adenovirus backbone. Kidney tubule cells were transfected with plasmids using Nucleofector (Lonza, Basel, Switzerland) according to the manufacturer's protocol.
SGK1 kinase assay
A purified, recombinant GST-Snail fusion protein was kindly provided by Dr Zhou (Galveston, TX).17 Active SGK1 (Millipore, Billerica, MA) was incubated with GST-Snail as a substrate (Cell Signaling, Denvers, MA) and in a volume of 30 μl kinase buffer (25 mmol/l Tris-HCl, pH 7.5, 5 mm β-glycerophosphate, 2 mmol/l dithiothreitol, 0.1 mmol/l Na3VO4, 10 mmol/l MgCl2, 100 μmol/l ATP and 8 μCi [γ-32P]ATP) for 10 min at 30 °C. The recombinant of GST-GSK-3β (Millipore) was used as a positive control for SGK1 kinase activity.17 GSK-3β was also immunoprecipitated using anti-GSK-3β antibody (Cell Signaling) from 500–1000 μg cell lysates, and incubated with GST-Snail. Kinase reactions were carried out.17 The reactions were stopped by adding sodium dodecyl sulfate (SDS) sample buffer and heating for 5 min at 95 °C. A volume of 20 μl of the sample was loaded on 10% SDS-polyacrylamide gel electrophoresis gels, separated, and the level of Snail phosphorylation was determined by autoradiography. Part of the reaction samples was used for western blot to assess Snail and SGK1 levels.
Cyclic stretch
Kidney tubular cells were plated on silicone elastomer-bottomed or collagen-coated plates. After achieving 90% confluence, cells were subjected to a cyclic mechanical stretch with a computer-controlled mechanical Strain Unit (Flexcell 4000) set at cyclic (60 cycles/min) stretch to yield a uniform deformation with 15% elongation of the elastomer-bottomed plates.51
Chromatin immunoprecipitation assay
The mouse E-cadherin promoter sequence (accession number: AY566874) contains several Snail recognition and binding elements. These are two E-boxes (CACCTG) located at –157 and –213 bp, respectively, from the transcription start site (sequence from 1117 to 1122 and from 1173 to 1178). Chromosomal DNA from normal or stretched kidney tubular cells derived from WT or SGK1 KO mice was isolated using a QiGene DNA Purification kit (Qiagen, Valencia, CA, USA). The DNA was immunoprecipitated with anti-Snail antibody. The PCR reactions used primers that flanked the E-box sequence; upstream from 1081 to 1102 (5′-TGGAGGAAGTTGAGGGCCCTGC-3′) and downstream from 1196 to 1173 (5′-CGCAGAGGCTGCGGCCGCCAGGAG-3′). The expected size is 116 bp. DNA samples before immunoprecipitation were used as a template for input control.
Immunofluorescence staining
Briefly, cells were fixed in 4% paraformaldehyde in PBS for 10 min at room temperature. Cells were permeabilized in PBS containing 0.1% Triton X-100 and blocked by incubating in 5% bovine serum albumin for 30 min. Fixed cells were washed with PBS and incubated for 60 min at room temperature or overnight at 4 °C with primary antibodies. After washing three times, the cells were stained with Fluor secondary antibodies (Invitrogene, Carlsbad, CA, USA). Images were obtained using a deconvolution microscope with a Zeiss Plan Apochromat 63×, 1.4 NA objective lens (Oberkochen, Germany).
Immunohistochemistry
Renal morphology was examined in 10% neutral-buffered formalin-fixed, paraffin-embedded tissue sections after they were stained with periodic acid-Schiff or Masson's-modified trichrome. For histological analysis, kidneys were perfused as described.51 After removing paraffin, sections were incubated for 30 min in 3% H2O2 in methanol at room temperature, washed with PBS, and heated in a microwave in 10 mm citrate buffer (pH 6.0) for 20 min. Sections were blocked using 10% goat serum (Vector Laboratories, Burlingame, CA, USA) for 30 min, incubated with anti-α-SMA (Abcam, Cambridge, MA), anti-mouse type I, and type IV collagen (Southern Biotechnology, Birmingham, AL, USA), anti-Snail and fibronectin (Santa Cruz, Santa Cruz, CA, USA), or anti-SGK1 antibodies.52 The staining protocol was performed according to the ABC kits (Vector Lab). The signals were visualized using a peroxidase substrate kit (Vector Lab). The pictures were recorded using a Nikon light microscope and staining intensity analyzed using Nikon software (Nikon Inc., Melville, NY, USA).
Western blot analysis
Protein from cells and kidney tissues was extracted with radio immunoprecipation assay lysis buffer (1% Nonidet P-40, 0.1% SDS, 1 mm phenylmethylsulfonyl fluoride, 0.5% sodium deoxycholate, 1 mm sodium orthovanadate, and 1 mm sodium fluoride in PBS). After determining the protein concentrations, 20 μg of the protein was mixed with an equal amount of 2 × SDS loading buffer (100 mmol/l Tris-HCl, 4% SDS, 20% glycerol and 0.2% bromophenol blue) for western blot analysis as described.51,53 The signals were visualized using Odyssey image system (Li-COR, Lincoln, NE, USA).
Real-time RT-PCR
Total RNA from control and UUO kidneys were isolated using the RNeasy kit, according to the manufacturer's instructions (Qiagen, Valencia, CA, USA). cDNAs were synthesized and real-time PCRs were run with the Opticon real-time PCR machine (MJ Research, Waltham, MA, USA). The specificity of real-time PCR was confirmed using routine agarose gel electrophoresis and melting-curve analysis; GAPDH was used as an internal standard. The primers used were mouse SGK1: forward 5′-TTCGTTAGCCTTTGGTGGAGTTGC-3′; reverse 5′-AGCACCACGTTGGAAGGAAGAGAA-3′; and GAPDH forward 5′-AGTGGGAGTTGCTGTTGAAATC-3′, reverse 5′-TGCTGAGTATGTCGTGGAGTCTA-3′.
Ribonuclease protection assay
SGK1 and L32 RNA probes were synthesized and labeled with α-32P-CTP.51 RNA was collected and purified from kidney tubular cells after exposure to mechanical stretch or TGF-β. The hybridization, RNase digestion, and precipitation were carried out according to the manufacturer's protocols (Torrey Pines Biolabs, East Orange, NJ, USA). Band intensities were normalized to L32 house keeping gene.
Statistics
Results were expressed as the mean±standard deviation. Student's unpaired t-test and an analysis of multiple variances by Scheff method was used for statistical comparison. P<0.05 was considered statistically significant.
ACKNOWLEDGMENTS
We acknowledge Dr William E Mitch for his inspirational support. This study was supported by AHA grant (10SDG2780009 to JC) and grants from the National Institutes of Health R37 DK37175, a 973 project of the Chinese Ministry of Science and Technology (2009CB522205) and from the National Science Foundation of China (30888004), and grants from the Deutsche Forschungsgemeinschaft (GRK 1302, SFB773).
Footnotes
DISCLOSURE
All the authors declared no competing interests.
REFERENCES
- 1.Grande MT, Lopez-Novoa JM. Fibroblast activation and myofibroblast generation in obstructive nephropathy. Nat Rev Nephrol. 2009;5:319–328. doi: 10.1038/nrneph.2009.74. [DOI] [PubMed] [Google Scholar]
- 2.Bascands JL, Schanstra JP. Obstructive nephropathy: insights from genetically engineered animals. Kidney Int. 2005;68:925–937. doi: 10.1111/j.1523-1755.2005.00486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chevalier RL. Obstructive nephropathy: towards biomarker discovery and gene therapy. Nat Clin Pract Nephrol. 2006;2:157–168. doi: 10.1038/ncpneph0098. [DOI] [PubMed] [Google Scholar]
- 4.Klahr S, Morrissey J. Obstructive nephropathy and renal fibrosis. Am J Physiol Renal Physiol. 2002;283:F861–F875. doi: 10.1152/ajprenal.00362.2001. [DOI] [PubMed] [Google Scholar]
- 5.Ricardo SD, Ding G, Eufemio M, et al. Antioxidant expression in experimental hydronephrosis: role of mechanical stretch and growth factors. Am J Physiol Renal Physiol. 1997;272:F789–F798. doi: 10.1152/ajprenal.1997.272.6.F789. [DOI] [PubMed] [Google Scholar]
- 6.Sato M, Muragaki Y, Saika S, et al. Targeted disruption of TGF-{beta}1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J Clin Invest. 2003;112:1486–1494. doi: 10.1172/JCI19270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coleman CM, Minor JJ, Burt LE, et al. Angiotensin AT1-receptor inhibition exacerbates renal injury resulting from partial unilateral ureteral obstruction in the neonatal rat. Am J Physiol Renal Physiol. 2007;293:F262–F268. doi: 10.1152/ajprenal.00071.2007. [DOI] [PubMed] [Google Scholar]
- 8.Chevalier RL, Thornhill BA, Wolstenholme JT. Renal cellular response to ureteral obstruction: role of maturation and angiotensin II. Am J Physiol. 1999;277(Part 2):F41–F47. doi: 10.1152/ajprenal.1999.277.1.F41. [DOI] [PubMed] [Google Scholar]
- 9.Lan HY, Mu W, Tomita N, et al. Inhibition of renal fibrosis by gene transfer of inducible Smad7 using ultrasound-microbubble system in rat UUO model. J Am Soc Nephrol. 2003;14:1535–1548. doi: 10.1097/01.asn.0000067632.04658.b8. [DOI] [PubMed] [Google Scholar]
- 10.Niessen CM, Gottardi CJ. Molecular components of the adherens junction. Biochim Biophys Acta. 2008;1778:562–571. doi: 10.1016/j.bbamem.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheng W, Wang G, La Pierre DP, et al. Versican mediates mesenchymalepithelial transition. Mol Biol Cell. 2006;17:2009–2020. doi: 10.1091/mbc.E05-10-0951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guaita S, Puig I, Franci C, et al. Snail induction of epithelial to mesenchymal transition in tumor cells is accompanied by MUC1 repression and ZEB1 expression. J Biol Chem. 2002;277:39209–39216. doi: 10.1074/jbc.M206400200. [DOI] [PubMed] [Google Scholar]
- 13.Batlle E, Sancho E, Franci C, et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 14.Medici D, Hay ED, Olsen BR. Snail and Slug promote epithelialmesenchymal transition through {beta}-catenin-T-cell factor-4-dependent expression of transforming growth factor-{beta}3. Mol Biol Cell. 2008;19:4875–4887. doi: 10.1091/mbc.E08-05-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kokudo T, Suzuki Y, Yoshimatsu Y, et al. Snail is required for TGF{beta}-induced endothelial-mesenchymal transition of embryonic stem cell-derived endothelial cells. J Cell Sci. 2008;121:3317–3324. doi: 10.1242/jcs.028282. [DOI] [PubMed] [Google Scholar]
- 16.Ivanova L, Butt MJ, Matsell DG. Mesenchymal transition in kidney collecting duct epithelial cells. Am J Physiol Renal Physiol. 2008;294:F1238–F1248. doi: 10.1152/ajprenal.00326.2007. [DOI] [PubMed] [Google Scholar]
- 17.Zhou BP, Deng J, Xia W, et al. Dual regulation of Snail by GSK-3[beta]-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol. 2004;6:931. doi: 10.1038/ncb1173. [DOI] [PubMed] [Google Scholar]
- 18.Masszi A, Fan L, Rosivall L, et al. Integrity of cell-cell contacts is a critical regulator of TGF-{beta}1-induced epithelial-to-myofibroblast transition: role for {beta}-catenin. Am J Pathol. 2004;165:1955–1967. doi: 10.1016/s0002-9440(10)63247-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peinado H, Quintanilla M, Cano A. Transforming growth factor {beta}-1 induces snail transcription factor in epithelial cell lines: mechanisms for epithelial mesenchymal transitions. J Biol Chem. 2003;278:21113–21123. doi: 10.1074/jbc.M211304200. [DOI] [PubMed] [Google Scholar]
- 20.Dominguez D, Montserrat-Sentis B, Virgos-Soler A, et al. Phosphorylation regulates the subcellular location and activity of the snail transcriptional repressor. Mol Cell Biol. 2003;23:5078–5089. doi: 10.1128/MCB.23.14.5078-5089.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Larue L, Bellacosa A. Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene. 2005;24:7443–7454. doi: 10.1038/sj.onc.1209091. [DOI] [PubMed] [Google Scholar]
- 22.Surendran K, Schiavi S, Hruska KA. Wnt-dependent beta-catenin signaling is activated after unilateral ureteral obstruction, and recombinant secreted frizzled-related protein 4 alters the progression of renal fibrosis. J Am Soc Nephrol. 2005;16:2373–2384. doi: 10.1681/ASN.2004110949. [DOI] [PubMed] [Google Scholar]
- 23.Firestone GL, Giampaolo JR, O'Keeffe BA. Stimulus-dependent regulation of serum and glucocorticoid inducible protein kinase (SGK) transcription, subcellular localization and enzymatic activity. Cell Physiol Biochem. 2003;13:1–12. doi: 10.1159/000070244. [DOI] [PubMed] [Google Scholar]
- 24.Verrey F, Loffing J, Zecevic M, et al. SGK1: aldosterone-induced relay of Na+ transport regulation in distal kidney nephron cells. Cell Physiol Biochem. 2003;13:21–28. doi: 10.1159/000070246. [DOI] [PubMed] [Google Scholar]
- 25.Lang F, Bohmer C, Palmada M, et al. (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol Rev. 2006;86:1151–1178. doi: 10.1152/physrev.00050.2005. [DOI] [PubMed] [Google Scholar]
- 26.Lang F, Klingel K, Wagner CA, et al. Deranged transcriptional regulation of cell-volume-sensitive kinase hSGK in diabetic nephropathy. Proc Natl Acad Sci USA. 2000;97:8157–8162. doi: 10.1073/pnas.97.14.8157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.BelAiba RS, Djordjevic T, Bonello S, et al. The serum- and glucocortico-idinducible kinase Sgk-1 is involved in pulmonary vascular remodeling: role in redox-sensitive regulation of tissue factor by thrombin. Circ Res. 2006;98:828–836. doi: 10.1161/01.RES.0000210539.54861.27. [DOI] [PubMed] [Google Scholar]
- 28.Kumar JM, Brooks DP, Olson BA, et al. Sgk, a putative serine/threonine kinase, is differentially expressed in the kidney of diabetic mice and humans. J Am Soc Nephrol. 1999;10:2488–2494. doi: 10.1681/ASN.V10122488. [DOI] [PubMed] [Google Scholar]
- 29.Klingel K, Warntges S, Bock J, et al. Expression of cell volume-regulated kinase h-sgk in pancreatic tissue. Am J Physiol Gastrointest Liver Physiol. 2000;279:G998–G1002. doi: 10.1152/ajpgi.2000.279.5.G998. [DOI] [PubMed] [Google Scholar]
- 30.Stevens VA, Saad S, Chen XM, et al. The interdependence of EGF-R and SGK-1 in fibronectin expression in primary kidney cortical fibroblast cells. Int J Biochem Cell Biol. 2007;39:1047–1054. doi: 10.1016/j.biocel.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 31.Vallon V, Wyatt AW, Klingel K, et al. SGK1-dependent cardiac CTGF formation and fibrosis following DOCA treatment. J Mol Med. 2006;84:396–404. doi: 10.1007/s00109-005-0027-z. [DOI] [PubMed] [Google Scholar]
- 32.Busjahn A, Luft FC. Twin studies in the analysis of minor physiological differences between individuals. Cell Physiol Biochem. 2003;13:51–58. doi: 10.1159/000070249. [DOI] [PubMed] [Google Scholar]
- 33.Luft FC. SGK1 survival through various lives may save us all. J Mol Med. 2007;85:657–659. doi: 10.1007/s00109-007-0224-z. [DOI] [PubMed] [Google Scholar]
- 34.Dieter M, Palmada M, Rajamanickam J, et al. Regulation of glucose transporter SGLT1 by ubiquitin ligase Nedd4-2 and kinases SGK1, SGK3, and PKB. Obes Res. 2004;12:862–870. doi: 10.1038/oby.2004.104. [DOI] [PubMed] [Google Scholar]
- 35.Schwab M, Lupescu A, Mota M, et al. Association of SGK1 gene polymorphisms with type 2 diabetes. Cell Physiol Biochem. 2008;21:151–160. doi: 10.1159/000113757. [DOI] [PubMed] [Google Scholar]
- 36.Bachelder RE, Yoon S-O, Franci C, et al. Glycogen synthase kinase-3 is an endogenous inhibitor of Snail transcription: implications for the epithelial-mesenchymal transition. J Cell Biol. 2005;168:29–33. doi: 10.1083/jcb.200409067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Yang J, Dai C, et al. Role for integrin-linked kinase in mediating tubular epithelial to mesenchymal transition and renal interstitial fibrogenesis. J Clin Invest. 2003;112:503–516. doi: 10.1172/JCI17913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang Z, Rayala S, Nguyen D, et al. Pak1 phosphorylation of snail, a master regulator of epithelial-to-mesenchyme transition, modulates Snail's subcellular localization and functions. Cancer Res. 2005;65:3179–3184. doi: 10.1158/0008-5472.CAN-04-3480. [DOI] [PubMed] [Google Scholar]
- 39.Li Y, Dai C, Wu C, et al. PINCH-1 promotes tubular epithelial-to-mesenchymal transition by interacting with integrin-linked kinase. J Am Soc Nephrol. 2007;18:2534–2543. doi: 10.1681/ASN.2007030315. [DOI] [PubMed] [Google Scholar]
- 40.Dong LQ, Liu F. PDK2: the missing piece in the receptor tyrosine kinase signaling pathway puzzle. Am J Physiol Endocrinol Metab. 2005;289:E187–E196. doi: 10.1152/ajpendo.00011.2005. [DOI] [PubMed] [Google Scholar]
- 41.Webster MK, Goya L, Ge Y, et al. Characterization of sgk, a novel member of the serine/threonine protein kinase gene family which is transcriptionally induced by glucocorticoids and serum. Mol Cell Biol. 1993;13:2031–2040. doi: 10.1128/mcb.13.4.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park J, Leong ML, Buse P, et al. Serum and glucocorticoid-inducible kinase (SGK) is a target of the PI 3-kinase-stimulated signaling pathway. EMBO J. 1999;18:3024–3033. doi: 10.1093/emboj/18.11.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lang F, Cohen P. Regulation and physiological roles of serum- and glucocorticoid-induced protein kinase isoforms. Sci STKE. 2001;13:RE17. doi: 10.1126/stke.2001.108.re17. [DOI] [PubMed] [Google Scholar]
- 44.Friedrich B, Warntges S, Klingel K, et al. Up-regulation of the human serum and glucocorticoid-dependent kinase 1 in glomerulonephritis. Kidney Blood Press Res. 2002;25:303–307. doi: 10.1159/000066794. [DOI] [PubMed] [Google Scholar]
- 45.Waerntges S, Klingel K, Weigert C, et al. Excessive transcription of the human serum and glucocorticoid dependent kinase hSGK1 in lung fibrosis. Cell Physiol Biochem. 2002;12:135–142. doi: 10.1159/000063790. [DOI] [PubMed] [Google Scholar]
- 46.Khan ZA, Barbin YP, Farhangkhoee H, et al. Glucose-induced serum- and glucocorticoid-regulated kinase activation in oncofetal fibronectin expression. Biochem Biophys Res Commun. 2005;329:275–280. doi: 10.1016/j.bbrc.2005.01.135. [DOI] [PubMed] [Google Scholar]
- 47.Shelly C, Herrera R. Activation of SGK1 by HGF, Rac1 and integrin-mediated cell adhesion in MDCK cells: PI-3K-dependent and -independent pathways. J Cell Sci. 2002;115:1985–1993. doi: 10.1242/jcs.115.9.1985. [DOI] [PubMed] [Google Scholar]
- 48.Cano A, Perez-Moreno MA, Rodrigo I, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 49.He W, Dai C, Li Y, et al. Wnt/{beta}-catenin signaling promotes renal interstitial fibrosis. Journal Title. 2009:765–776. doi: 10.1681/ASN.2008060566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wulff P, Vallon V, Huang DY, et al. Impaired renal Na(+) retention in the sgk1-knockout mouse. J Clin Invest. 2002;110:1263–1268. doi: 10.1172/JCI15696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheng J, Du J. Mechanical stretch simulates proliferation of venous smooth muscle cells through activation of the insulin-like growth factor-1 receptor. Arterioscler Thromb Vasc Biol. 2007;27:1744–1751. doi: 10.1161/ATVBAHA.107.147371. [DOI] [PubMed] [Google Scholar]
- 52.Zhang L, Cui R, Cheng X, et al. Antiapoptotic effect of serum and glucocorticoid-inducible protein kinase is mediated by novel mechanism activating I{kappa}B kinase. Cancer Res. 2005;65:457–464. [PubMed] [Google Scholar]
- 53.Cheng J, Cui R, Chen C-H, et al. Oxidized low-density lipoprotein stimulates p53-dependent activation of proapoptotic bax leading to apoptosis of differentiated endothelial progenitor cells. Endocrinology. 2007;148:2085–2094. doi: 10.1210/en.2006-1709. [DOI] [PubMed] [Google Scholar]