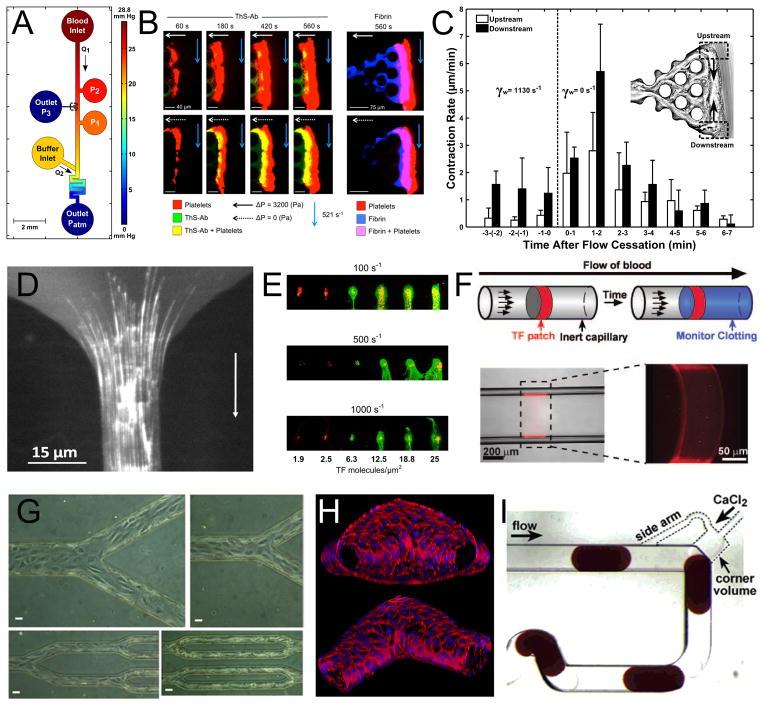
Fig. 3. Microfluidic Devices of Coagulation Research.
A, A microfluidic model of bleeding designed for the study of thrombus permeability under flow. The device has integrated ports for localization of collagen and TF to the channel wall, pressure sensors, and buffer flow for precise pressure control. Blood flow (from top to bottom) will seep through the collagen scaffold connected to Outlet P3 until a platelet plug is formed. Reproduced with permission from Reference 97. Copyright American Heart Association, Inc. B, Epi-fluorescence microscopy performed at the collagen/TF scaffold of the device pictured in A reveals a large platelet aggregate (red) formed during perfusion which also stains positive for thrombin using a novel thrombin bio-sensor. Comparison of the top to bottom rows in which blood flow is allowed (top) or not allowed (bottom) to seep through Outlet P3 reveals that thrombin permeation into the clot is enhanced when it is not convected away. These clots also stain positive for fibrin (right). Reproduced with permission from Reference 96. Copyright International Society on Thrombosis and Hemostasis. C, Thrombus contraction was also observed in the microfluidic bleeding model. Here it is demonstrated that ~1 min after flow cessation the rate of clot retraction, measured by tracking the movement of trapped fluorescent beads, was 3-fold enhanced. Reproduced with permission from Reference 97. Copyright American Heart Association, Inc. D, A microfluidic model of stenosis reveals long fiber bundles of von Willebrand factor forming on a collagen type 1 surface under plasma flow at shear rates >30,000 s−1. Reproduced with permission from Reference 101. Copyright American Heart Association, Inc. E, Microcontact printing of collagen and varying amounts of tissue factor reveals a steep threshold for fibrin generation (green) under whole blood flow at varying shear rates in a parallel plate flow chamber construct. Platelets are labeled in red. This research was originally published in Reference 56. Copyright the American Society of Hematology. F, TF has also been patterned into microcapillary flow models using photolithographic techniques. The drawing illustrates that a soluble fluorescent reporter of thrombin will be activated downstream of the procoagulant patch. Reproduced with permission from Reference 55. Copyright Wolters Kluwer Health. G, The culture of confluent monolayers of endothelium has been achieved in microfluidic devices of PDMS (Republished with permission of the American Society for Clinical Investigation, from Reference 27) as well as on 3-dimensional collagen scaffolds (H). The in vitro vessels pictured in H were designed for studies involving permeability, angiogenesis, as well as thrombus formation after endothelial activation. Reproduced with permission from Reference 29. I, The microfluidic droplet reactor pictured here was designed to study clotting time in the presence of various inhibitors. Plugs of citrated whole blood enter at the top to which CaCl2 is added. The plug at the bottom left is entering the mixing region after which clotting will be assessed in a downstream region of the channel. Reprinted with permission from Reference 92. Copyright 2006 American Chemical Society.