Abstract
Before contacting host tissues, invading pathogens directly or indirectly interact with host microbiota, but the effects of such interactions on the initial stages of infection are poorly understood. Bordetella pertussis is highly infectious among humans but requires large doses to colonize rodents, unlike a closely related zoonotic pathogen, Bordetella bronchiseptica, raising important questions about the contributions of bacterial competition to initial colonization and host selection. We observed that <100 colony-forming units (CFU) of B. bronchiseptica efficiently infected mice and displaced culturable host microbiota, whereas 10 000 CFU of B. pertussis were required to colonize murine nasal cavities and did not displace host microorganisms. Bacteria isolated from murine nasal cavities but not those from the human lower respiratory tract limited B. pertussis growth in vitro, indicating that interspecies competition may limit B. pertussis colonization of mice. Further, a broad-spectrum antibiotic treatment delivered before B. pertussis inoculation reduced the infectious dose to <100 CFU, and reintroduction of single Staphylococcus or Klebsiella species was sufficient to inhibit B. pertussis colonization of antibiotic-treated mice. Together, these results reveal that resident microorganisms can prevent B. pertussis colonization and influence host specificity, and they provide rationale for manipulating microbiomes to create more-accurate animal models of infectious diseases.
Keywords: microbiome, Bordetella, antibiotics, whooping cough, pathogen evolution, host adaptation, interspecies competition, bacterial competition
Bordetella pertussis is the major causative agent of whooping cough, which continues to persist in both highly vaccinated and unvaccinated populations worldwide. The World Health Organization estimates that 20–40 million whooping cough cases occur globally per year, resulting in >400 000 deaths each year [1, 2]. Although B. pertussis vaccines have been highly effective [3], the annual incidence of B. pertussis has recently increased even in highly vaccinated populations [4]. In the United States, >25 000 cases have been reported every year since 2005 [5, 6]. However, efforts to better control and eliminate whooping cough have been hindered by the lack of experimental models to investigate how B. pertussis initially colonizes individuals and efficiently transmits between susceptible hosts.
B. pertussis is a member of the classical bordetellae, a group of closely related respiratory pathogens that also includes the widespread zoonotic pathogen Bordetella bronchiseptica. Although B. pertussis appears to be host restricted to humans, B. bronchiseptica has a very diverse host range [7] and can cause a wide range of diseases, from asymptomatic colonization to lethal pneumonia [8]. Interestingly, B. pertussis appears to have evolved from a B. bronchiseptica–like progenitor strain through genome rearrangements and degradation [9, 10]. In a murine infection model, both species colonize the lower respiratory tract when administered in a high-dose, high-volume regimen of 5 × 105 colony-forming units (CFU) in 50 µL [11–13]. However, even though these closely related pathogens share about 98.6% sequence similarity among orthologous genes (J. Park and E. T. Harvill, personal communication) and have been referred to as subspecies, their median infectious doses (ID50) in mice differ significantly, from <5 CFU of B. bronchiseptica to >10 000 CFU of B. pertussis [7]. Additionally, B. bronchiseptica remains in the murine upper respiratory tract for the life of the animal [11, 14], while even high doses of B. pertussis are cleared from the nasal cavity within 1 month after inoculation [11, 15, 16].
In this study, we initially aimed to understand why B. pertussis fails to colonize the murine nasal cavity as effectively as B. bronchiseptica. Because both B. pertussis and B. bronchiseptica have been shown to adhere to murine epithelial cell lines in vitro and both species can colonize the nasal cavity at high levels following high-dose inoculation, we considered that differences in colonization were likely not exclusive to effective adherence to nasal cavity tissue [17–21]. Next, we considered that distinct interactions with the host immune system could affect initial colonization. However, both species have been observed to effectively colonize a similar array of immunodeficient mice, suggesting that differential immune responses may not mediate these differences [12, 13, 22–24]. Because the nasal cavity is known to harbor a complex community of microbiota, we also considered the possibility that interactions with resident microbes could contribute to these differences in colonization. Both immune-mediated and direct mechanisms of competition can impact pathogen success within a host [25–28]. Recent studies have indicated that Bordetella species contain several mechanisms for interbacterial competition, including bacteriophages, a type VI secretion system, and, potentially, contact-dependent growth inhibition (CDI) [29–31]. On the basis of these observations, we hypothesized that the ability to compete with resident host organisms may mediate the ability of pathogens to successfully colonize a host. Here, we demonstrate that removing resident microorganisms from the nasal cavity allows B. pertussis to efficiently colonize the murine nasal cavity and that reintroduction of a single nasal cavity bacterial species was sufficient to block B. pertussis colonization in mice, demonstrating that interactions with nasal cavity flora can significantly contribute to infection dynamics.
MATERIAL AND METHODS
Ethics Statement
This study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee at The Pennsylvania State University, University Park [protocol 31 297, Bordetella-Host Interaction]). All animals were anesthetized using isoflurane or euthanized using carbon dioxide inhalation, to minimize animal suffering.
Bacterial Strains
B. pertussis strain 536, a streptomycin-resistant derivative of the sequenced strain Tohama I and B. bronchiseptica strain RB50 are described elsewhere [32, 33]. Bordetellae were maintained on Bordet-Gengou agar (Difco) with 10% sheep blood (Hema Resources) and 20 µg/mL streptomycin (Sigma). Host bacterial isolates were obtained by culture on lysogeny broth agar or blood agar (BD Biosciences) supplemented with 5% sheep blood (Hema Resources). B. bronchiseptica and host microbiota species were grown on agar for 48 hours at 37°C, while B. pertussis was grown for 5 days at 37°C. To obtain liquid cultures, bordetellae were grown overnight to exponential phase in Stainer-Scholte broth while shaking at 37°C, whereas host microbiota isolates were similarly grown in lysogeny broth (BD Biosciences).
Mouse Experiments
C57BL/6 wild-type mice were obtained from Jackson Laboratories (Bar Harbor, ME) and were housed and bred in a specific-pathogen-free facility at The Pennsylvania State University. All animal experiments were performed according to institutional guidelines and completed essentially as previously described [14, 33, 34]. Briefly, groups of 4 mice were inoculated by gently pipetting the indicated number of Bordetella species or host microorganisms suspended in 10 µL of sterile phosphate-buffered saline onto the external nares of anesthetized mice to mimic natural infection. Three days after inoculation, mice were euthanized using CO2, and the nasal cavity was excised, homogenized, and plated on Bordet-Gengou agar, to identify quantities of bordetellae, or blood agar and lysogeny broth agar, to identify quantities of host microorganisms. Antibiotic treatment was achieved by gently pipetting 45 µg of enrofloxacin (Baytril) in 10 µL of phosphate-buffered saline on 3 separate occasions 12 hours apart onto the external nares of anesthetized mice, and mice were then inoculated with bacteria at least 8 hours later.
Host Species Identification
All nasal cavity host microorganisms cultured on agar were described morphologically, isolated in pure culture, and preserved in 20% glycerol (Sigma) at −80°C. DNA extraction using the QIAmp DNA Mini Kit (Qiagen) was performed on each distinct organism, and the 16S ribosomal RNA (rRNA) V2 region was amplified using 16SF (AGAGTTTGATCATGGCTCAG) and 16SR (AAGGAGGTGATCCAACC) primers under the following conditions: 94°C for 5 minutes; 95°C for 30 seconds, 56°C for 1 minute, and 72°C for 1.5 minutes, 30 times; and 72°C for 8 minutes [35]. Following amplification, the approximately 1500-bp polymerase chain reaction products were isolated and purified using MinElute Gel Extraction Kit (Qiagen) as per the manufacturer's instructions. Sequencing of each 16S rRNA–encoding region was completed at The Pennsylvania State University Genomics Core Facility. Both forward and reverse sequences were aligned via MEGA4 [36], and the consensus sequence was submitted to the National Center for Biotechnology Information BLAST (Basic Local Alignment Search Tool) for identification [37]. The top known hit was used for identification with a cutoff of 70% homology.
16S rRNA Amplicon Sequencing
Animal dissection and DNA isolation was performed from whole nasal cavities in a UV-sterilized hood with DNA-zap (Ambion)–treated instruments and DNA- and RNA-free filtered pipette tips. The BiOstatic formalin-fixed paraffin-embedded tissue DNA isolation kit (Mo Bio Laboratories) was used to isolate total DNA, with the following modifications: the samples were left at 55°C overnight rather than for 1 hour (step 3), and step 4 was omitted. At The Pennsylvania State University Core Genomic facility, 454 amplicon amplification, library preparation, and sequencing were performed. Bar-coded samples were pooled and sequenced together, achieving approximately 20 000 reads/sample. Sequences were initially analyzed by mothur [38] and identified by BLAST according to the Greengenes database [38]. Sequences were then analyzed via MEGAN (MEtaGenome ANalyzer), producing phylogenetic classification and relationships to known National Center for Biotechnology Information taxonomic trees [39].
In Vitro Bacterial Competition Experiments
Staphylococcus saprophyticus, Staphylococcus cohnii, Staphylococcus xylosus, Kytococcus species, Klebsiella species, Rhizobium species, Streptococcus oralis, Streptococcus mitis, Neisseria subflava, Corynebacterium species, and B. pertussis cultures were grown to exponential phase. By use of the optical densities to estimate culturable CFUs, all cultures were then all diluted to 1 × 107 CFU/mL in Stainer-Scholte liquid medium. Competition between each host microorganism and B. pertussis was created by mixing each host species with an equal number of B. pertussis organisms and incubating the mixture in Stainer-Scholte at 37°C for 32 hours while shaking. B. pertussis and each microbiota species were additionally grown alone in Stainer-Scholte medium, starting with 1 × 107 CFU/mL as a control. Each coculture and control was performed in triplicate and plated on Bordet-Gengou agar with 60 ng/mL streptomycin and blood agar at 0, 2, 4, 8, 24, and 36 hours. The plates were incubated for 48 hours (microbiota) or 5 days (B. pertussis) at 37°C, and resulting colonies were counted. Statistical analysis was performed by completing a Student t test, and a P value of ≥ .05 was considered statistically significant.
RESULTS
B. pertussis Requires 10 000 CFU to Colonize the Murine Nasal Cavity
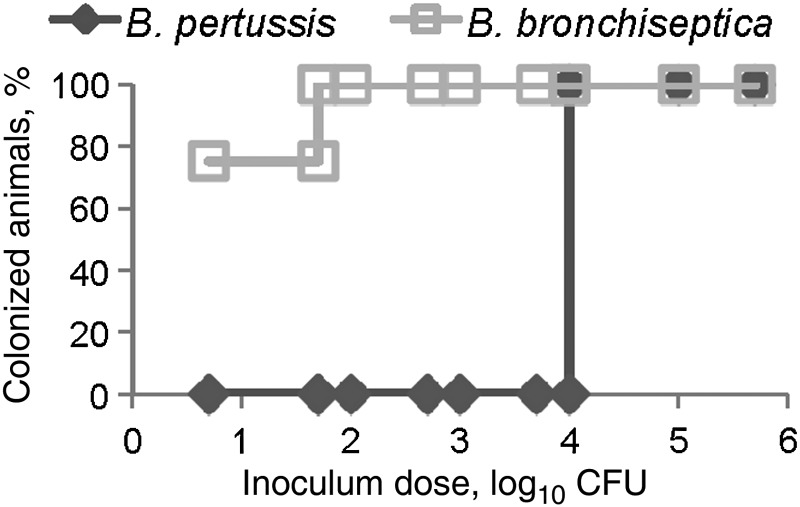
Although high-dose, high-volume delivery of B. bronchiseptica and B. pertussis leads to efficient growth in the murine nasal cavity [16], these pathogens have not been compared at lower, more natural doses necessary to study the mechanisms of efficient initial colonization. To compare the infectious doses of these 2 organisms, C57BL/6 wild-type mice were inoculated with 10 µL of phosphate-buffered saline containing 5, 50, 100, 500, 1 × 103, 5 × 103, 1 × 104, 1 × 105, or 1 × 106 CFU of B. pertussis strain 536 or B. bronchiseptica strain RB50, and bacterial numbers were determined 3 days after inoculation, capturing several of the important stages during the early colonization phase of infection. A dose of 5 CFU of B. bronchiseptica in a 10-µL inoculum was sufficient to colonize the nasal cavity of two-thirds of the mice, whereas 10 000 CFU of B. pertussis in 10 µL was required to recover any B. pertussis 3 days after inoculation (Figure 1). A recent human isolate of B. pertussis, strain STO1-CHOC-0008 (obtained from a Collaborative Pediatric Critical Care Research Network), also failed to colonize mice at a low dose (data not shown). These results suggest that B. bronchiseptica, a natural murine pathogen, has mechanisms to efficiently colonize the murine nasal cavity that B. pertussis, a human-restricted pathogen, lacks.
Figure 1.
A total of 104 Bordetella pertussis are required to colonize the murine nasal cavity. Ten microliters of phosphate-buffered saline containing the indicated colony-forming units (CFU) of either Bordetella bronchiseptica (□) or B. pertussis (◊) was inoculated into groups of wild-type C57BL/6 mice. Nasal cavity colonization was assessed 3 days later and is presented as the percentage of colonized animals 3 days after inoculation.
B. bronchiseptica Can Displace Murine Microbiota In Vivo, Whereas B. pertussis Cannot
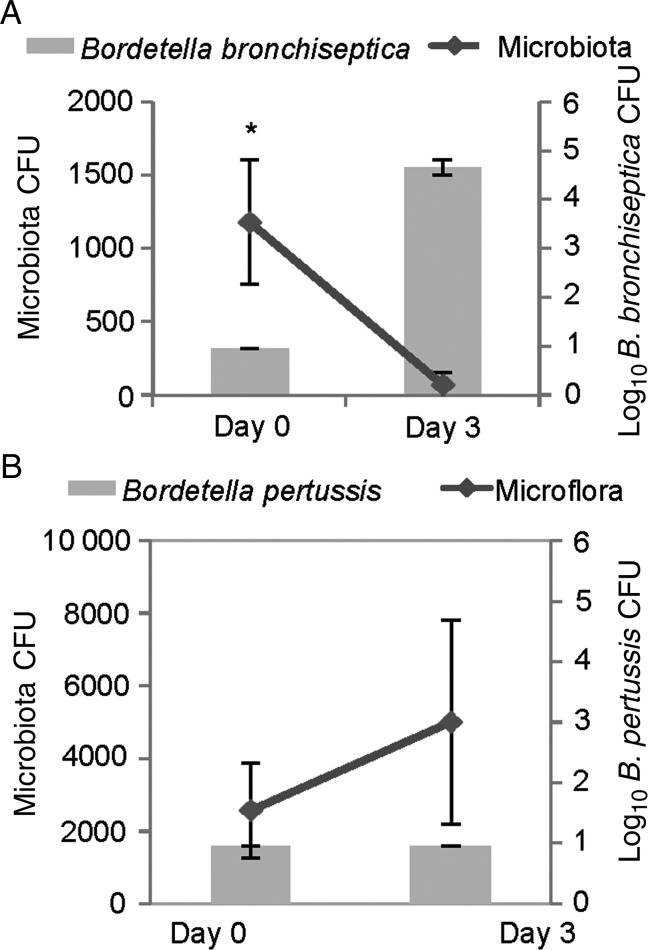
To examine Bordetella interactions with host microbiota in vivo, C57BL/6 littermates were cohoused for 5 weeks, and then half of the mice were inoculated with 100 CFU of B. bronchiseptica, whereas the other half were left uninfected. The same experiment was performed with B. pertussis. As before, B. bronchiseptica was able to colonize the upper respiratory tract at this low dose, whereas B. pertussis was not detected 3 days later in mice inoculated with this low dose (Figure 2A and 2B). Interestingly, B. bronchiseptica displaced culturable microbiota from the nasal cavities within 3 days after inoculation (Figure 2A), but B. pertussis did not (Figure 2B). This experiment identifies a key difference between these closely related species that correlates with their different ability to colonize mice, therefore presenting the hypothesis that competition with resident microbiota may mediate these differences.
Figure 2.
The widespread zoonotic pathogen Bordetella bronchiseptica can displace murine flora. Wild-type C57BL/6 mice were inoculated with 100 colony-forming units (CFU) in 10 µL of B. bronchiseptica strain RB50 (A) or Bordetella pertussis strain 536 (B). Host microbiota (lines) and bordetellae (bars) colonization were assessed before inoculation and 3 days after inoculation. Error bars represent SDs. *P < .05.
Murine Nasal Cavity Microbes Can Limit B. pertussis Growth In Vitro
To identify bacterial species that interact with B. pertussis within the upper respiratory tract, microbiota were cultured from murine nasal cavities on blood agar and identified by sequencing the 16S rRNA–encoding genome region. In these untreated and uninfected mice, 8 different culturable microorganisms were identified, including Staphylococcus, Kytococcus, Klebsiella, and Rhizobium species (Supplementary Table 1). A 16S rRNA amplicon sequencing approach was also used to identify all unculturable bacterial microorganisms present in the nasal cavity (Figure 3). In all, >80 different bacterial genera were identified using this approach, indicating that the nasal cavity of specific-pathogen-free mice is a diverse and microbe-rich environment.
Figure 3.
Over 80 different bacterial species are identified in the murine nasal cavity. Nasal cavities from uninfected C57BL/6 mice were obtained from our pathogen-free breeding facility. Total DNA was extracted from excised nasal cavities, and the 16S ribosomal RNA encoding region was amplified and sequenced via 454 sequencing to obtain >10 000 reads per animal. Sequences were identified by homology to known sequences in the Greengenes database, and evolutionary relationships between species were analyzed by creating phylogenetic trees in MEGAN, based on the National Center for Biotechnology Information bacterial database.
To examine competitive interactions between B. pertussis and isolated murine nasal cavity microbes, we assayed the in vitro growth of B. pertussis over time in the presence and absence of individual nasal cavity microorganisms. Stainer-Scholte medium was inoculated with 107 CFU of B. pertussis alone or together with either S. saprophyticus, Staphylococcus cohnii, S. xylosus, Klebsiella PAMU, Kytococcus species, or Rhizobium species and incubated with shaking at 37°C for 32 hours. B. pertussis alone grew to approximately 5 × 109 CFU/mL, but numbers were reduced by at least 90% when it was cocultured with Staphylococcus, Kytococcus, or Rhizobium species and by >99.9% when incubated with Klebsiella PAMU (Figure 4A). However, the growth of each host species was mostly unaffected by the presence of B. pertussis (Supplementary Figure 1A–F), although coculture with B. pertussis appeared to provide Rhizobium a slight growth advantage at 32 hours (Figure 4B). Together, these results suggest that murine nasal cavity bacteria are capable of inhibiting B. pertussis growth in vitro.
Figure 4.
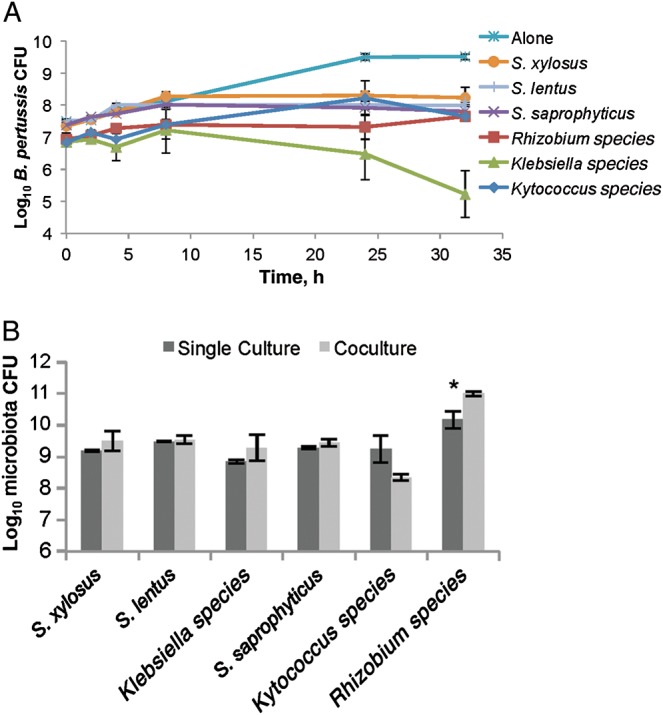
Murine nasal cavity flora inhibits Bordetella pertussis growth. B. pertussis was grown alone (star) or cocultured with either Staphylococcus saprophyticus (cross), Staphylococcus xylosus (circle), Staphylococcus lentus (line), Rhizobium (square), Kytococcus (diamond), or Klebsiella PAMU (triangle) in Stainer-Scholte media for 32 hours, and surviving B. pertussis colony-forming units (CFU) were determined by plating on Bordet-Gengou agar with 60 ng/mL streptomycin. *P > 0.05.
Human Lower Respiratory Tract Bacterial Isolates Do Not Limit B. pertussis Growth
Although earlier experiments demonstrated that murine nasal cavity flora can limit B. pertussis growth, B. pertussis is highly infectious and successfully colonizes the human lower respiratory tract, indicating that there may not be competitors there that can efficiently block its invasion and colonization. Therefore, we hypothesized that B. pertussis is able to successfully compete with microorganisms isolated from its only known environmental niche, the human lower respiratory tract. To test this hypothesis, lower respiratory tract microorganisms were cultured from human sputum, obtained from healthy, uninfected human subjects (Supplementary Table 1). These isolates were recovered on blood agar and later grown in the presence of B. pertussis in vitro, as done with the murine nasal cavity isolates. Interestingly, B. pertussis growth was unaffected by the presence of S. mitis, S. oralis, N. subflava, or Corynebacterium species for up to 48 hours, compared to B. pertussis grown alone (Figure 5A). However, S. oralis, N. subflava, and Corynebacterium species growth was lower after 48 hours when grown with B. pertussis than when each species was grown alone (Figure 5B), even though each species had a different growth rate (Supplementary Figure 2). S. mitis growth was unaffected by B. pertussis (Figure 5B). These data indicate that B. pertussis can successfully compete with these human lower respiratory tract isolates, suggesting that B. pertussis has retained mechanisms necessary to compete with bacterial species within its ecological niche.
Figure 5.
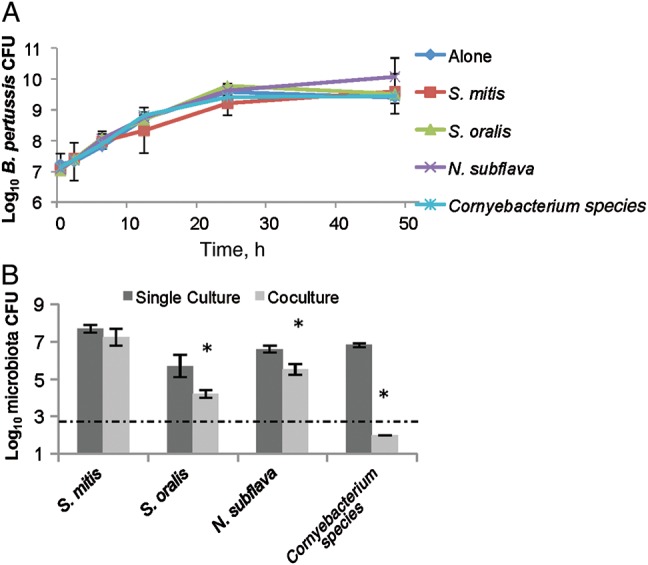
Bordetella pertussis outcompetes lower respiratory tract human isolates. B. pertussis was grown alone (diamond) or in the presence of Streptococcus mitis (square), Streptococcus oralis (triangle), Neisseria subflava (cross), and a Corynebacterium (star) isolate for up to 48 hours. Surviving B. pertussis were detected by plating on Bordet-Gengou agar with 60 ng/mL streptomycin (A). Remaining host microbiota from the cocultures (light) or when grown alone (dark) at 48 hours after inoculation were determined by plating on blood agar (B). *P > 0.05.
Murine Nasal Cavity Microbiota Inhibit B. pertussis Nasal Cavity Colonization
The observed competition with mouse nasal cavity microorganisms could potentially explain the failure of low doses of B. pertussis to colonize the mouse nasal cavity. To test this possibility, C57BL/6 mice were first intranasally treated with a broad-spectrum antibiotic, enrofloxacin, a bactericidal compound that affects both Gram-positive and Gram-negative species. Both antibiotic-treated and control mice were then inoculated with 100, 500, 1000, 5000, or 10 000 CFU B. pertussis, and bacterial numbers were determined 3 days later. A total of 10 000 CFU of B. pertussis were required to colonize mice with their microbiota still intact, as expected, but as few as 100 CFU of B. pertussis were sufficient to colonize mice previously treated with enrofloxacin (Figure 6). This finding indicates that bacterial communities present in the upper respiratory tract of mice can inhibit B. pertussis nasal cavity colonization.
Figure 6.
Baytril treatment reduced the Bordetella pertussis median infectious dose (ID50). Wild-type C57BL/6 mice were either treated 3 times with Baytril (light) or left untreated (dark). All mice were then inoculated with indicated doses of B. pertussis strain 536 in 10 µL, and levels of B. pertussis nasal cavity colonization were assessed 3 days after inoculation. Error bars represent SDs. Abbreviations: CFU, colony-forming units; ND, not detected.
Klebsiella or Staphylococcus Species Are Sufficient to Prevent B. pertussis Colonization
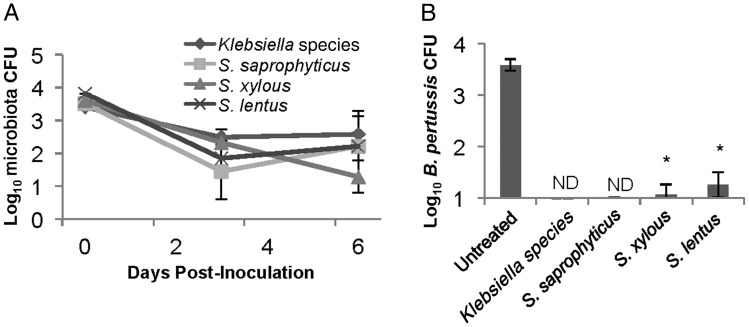
To determine whether single bacterial species are sufficient to block B. pertussis colonization, wild-type C57BL/6 mice were intranasally treated with enrofloxacin, and 3 days later 1000 CFU of Klebsiella PAMU, S. saprophyticus, S. lentus, or S. xylosus were administered to reintroduce individual microorganisms. Each of the other species was recovered from animals at numbers similar to those observed in untreated mice (Figure 7A). Then, 3 mice within these inoculated groups were additionally inoculated with 5000 CFU of B. pertussis, >10 times the infectious dose in antibiotic-treated mice. This inoculum resulted in approximately 1000 CFU of B. pertussis 3 days after inoculation in all antibiotic-treated mice. However, this dose was not sufficient to colonize antibiotic-treated mice in which Klebsiella or S. saprophyticus had been reintroduced (Figure 7B). Only tens of B. pertussis were detected in mice in which S. lentus or S. xylosus had been reintroduced (Figure 7B). These data indicate that single bacterial species from the murine nasal cavity are sufficient to inhibit B. pertussis colonization of the murine nasal cavity. Furthermore, levels of Klebsiella PAMU, S. saprophyticus, S. lentus, or S. xylosus were maintained at similar numbers after B. pertussis inoculation (Figure 7A), confirming that this human pathogen does not displace these species under these conditions.
Figure 7.
Inoculation with known microbiota inhibits Bordetella pertussis colonization. C57BL/6 mice were treated with enrofloxacin and then inoculated with 5000 colony-forming units (CFU) of Staphylococcus saprophyticus (square), Staphylococcus lentus (cross), Staphylococcus xylosus (triangle), or Klebsiella (diamond). A, Colonization of these organisms was monitored at 0 and 3 days after inoculation and at day 6 after inoculation, 3 days after inoculation of approximately 5000 CFU of B. pertussis. B, B. pertussis colonization on day 6 after inoculation of microbiota and 3 days after inoculation of B. pertussis was determined for each of the groups initially inoculated with individual host microbiota species. When B. pertussis was not detectable, the sample was labeled “ND.” *P > 0.05.
DISCUSSION
The earliest events in the colonization of a new host are critical limiting aspects of transmission, disease spread, and pathogenesis, but the microscopic scale in which these events occur make them difficult to observe and measure. Often, unnaturally high doses are often used to investigate infectious diseases in experimental models to achieve reproducibility, with very little consideration of how this affects initial colonization mechanisms, such as adherence, immune evasion, competition with other bacteria, and growth rates. The murine model of B. pertussis is among the best animal models of human diseases, in that it has been highly effective in revealing aspects of B. pertussis infection kinetics, immune response, and vaccination efficacy [40]. However, it has also been criticized for its use of relatively large inocula of approximately 5 × 105 CFU. Although its efficient growth within the respiratory tract and eventual clearance by immune mechanisms all closely resemble the infectious process in humans, the limiting steps during the earliest stages of colonization are poorly mimicked by the common high-dose inoculation regimens. In this study, low-dose, low-volume inoculations in mice are used to investigate how competition with microbiota alters host range and infectiousness, ultimately demonstrating that low-dose inoculations with B. pertussis can be successfully achieved if animals are first treated with enrofloxacin. Interestingly, large inoculation doses must be used in murine models to study other human pathogens, such as Pseudomonas, Burkholderia, and Streptococcus species. Therefore, it is plausible that host microorganisms also contribute to observed host specificity in other murine infection models.
Although we observed that B. bronchiseptica was capable of displacing murine nasal cavity flora while B. pertussis is only successful against lower respiratory tract flora, the mechanisms behind these interactions remain unclear. B. pertussis may lack factors necessary for competition against murine microbiota, but because several attempts to routinely isolate lower respiratory tract murine microorganisms failed, murine lower respiratory tract microorganisms have yet to be examined. Nevertheless, type VI secretion systems (T6SS) have been found to be required for microbial competition in Pseudomonas, Vibrio, and Serratia species [41–43], and colicins, phages, and CDI systems have been shown to mediate bacterial competition in Escherichia coli [31, 44, 45]. Our laboratory recently described a T6SS locus in B. bronchiseptica that may be involved in competition against murine nasal cavity flora and observed that this locus is missing in B. pertussis [46]. Interestingly, we find that B. pertussis contains genes annotated to encode colicins, phages, and potential CDI systems, but these mechanisms are apparently not sufficient to outcompete murine nasal cavity microorganisms, although they may be effective against human lower respiratory tract isolates [30]. Unique subsets or combinations of competition mechanisms may be required to simultaneously overcome multiple resident bacterial species to efficiently colonize a host.
Parkhill et al suggested that B. pertussis adaptation to humans correlates with a 25% loss of its genome, compared with the evolutionary progenitor-like strain B. bronchiseptica [10]. Until now, it has remained unclear how genome loss could result in host adaptation between 2 closely related strains that still maintain similar virulence factors. We speculate that B. pertussis, which appears to have a closed life cycle involving only acute infections and direct transmission from one human to another, may have lost many genes involved in interspecies competition outside of the human lower respiratory tract. In contrast, the ability of B. bronchiseptica to outcompete numerous host species is likely a critical factor to persistently colonize a wide range of mammals. The differential ability to compete with host microbiota could potentially explain the differences in host range, infectiousness, persistence, and/or infection strategy observed between these 2 closely related pathogens. Identifying differences in microbiota from different host species and organs and understanding how pathogens interact with these flora will elucidate key factors that contribute to initial colonization events that, in turn, will suggest novel ways to combat disease.
In this study, the resident species identified in the murine nasal cavity are wildly diverse, but many of these species are also commonly isolated from the upper respiratory tract in humans. Staphylococcus, Kytococcus, and Klebsiella species are routinely cultured from the human nasal cavity [47, 48], and therefore the interactions identified in this study between B. pertussis and nasal cavity flora may also occur in humans. Interestingly, B. pertussis can be difficult to culture from nasopharyngeal swab specimens from patients with pertussis-like symptoms, and polymerase chain reaction and serology must then be used to efficiently and reproducibly identify whooping cough–associated infections. Competition with nasal cavity organisms could limit B. pertussis growth in and isolation from the nasal cavity. Alternatively, B. pertussis could be more quickly displaced from the nasal cavity than from the lower respiratory tract, making it difficult to culture after symptoms present. Furthermore, our results suggest that disruption of resident nasal cavity microbiota may increase susceptibility to B. pertussis nasal cavity colonization. This could contribute to observations that infants, who likely lack a well-established nasal cavity microbiota, may be more susceptible than healthy adults to B. pertussis infections. Furthermore, these results may also suggest that administering broad-spectrum antibiotics to people could facilitate nasal cavity colonization of B. pertussis [47]. Nevertheless, this work provides a tractable experimental system in which to investigate the mechanisms that drive microbiome contributions to infectious diseases susceptibility.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank The Pennsylvania State University Core Genomics Facility, for completing the 454 amplicon sequencing presented in this article, and Laura Goodfield, for additional help with experimentation and interpretation of results.
Disclaimer. The of this article contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Financial support. This work was supported by the National Institutes of Health (grant GM083113 to E. T. H. and training grant T32AI074551 to S. J. M.) and the National Science Foundation Graduate Research Fellowship Program (to L. S. W.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization. Geneva: WHO; 2004. The world health report 2004—changing history. http://www.who.int/whr/2004/en/ Accessed 12 January 2012. [Google Scholar]
- 2.Tan T, Trindade E, Skowronski D. Epidemiology of pertussis. Pediatr Infect Dis J. 2005;24:S10–8. doi: 10.1097/01.inf.0000160708.43944.99. http://journals.lww.com/pidj/Fulltext/2005/05001/Epidemiology_of_Pertussis.3.aspx . [DOI] [PubMed] [Google Scholar]
- 3.Cherry JD. Historical review of pertussis and the classical vaccine. J Infect Dis. 1996;174:S259–63. doi: 10.1093/infdis/174.supplement_3.s259. [DOI] [PubMed] [Google Scholar]
- 4.Mielcarek N, Debrie A, Raze D, et al. Live attenuated B. pertussis as a single-dose nasal vaccine against whooping cough. PLoS Pathog. 2006;2:e65. doi: 10.1371/journal.ppat.0020065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Pertussis vaccines. The pink book: course textbook (May 2012) 2nd ed. Updated 20 September 2013. http://www.cdc.gov/vaccines/pubs/pinkbook/pert.html . Accessed 15 November 2013.
- 6.Pam D. Whooping cough makes global comeback. Lan Infect Dis. 2002;2:322. doi: 10.1016/s1473-3099(02)00308-0. [DOI] [PubMed] [Google Scholar]
- 7.Mattoo S, Cherry JD. Molecular pathogenesis, epidemiology, and clinical manifestations of respiratory infections due to Bordetella pertussis and other Bordetella subspecies. Clin Microbiol Rev. 2005;18:326–82. doi: 10.1128/CMR.18.2.326-382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodnow RA. Biology of Bordetella bronchiseptica. Microbiol Rev. 1980;44:722–38. doi: 10.1128/mr.44.4.722-738.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diavatopoulos DA, Cummings CA, Schouls LM, et al. Bordetella pertussis, the causative agent of whooping cough, evolved from a distinct, human-associated lineage of B. bronchiseptica. PLoS Pathog. 2005;1:e45. doi: 10.1371/journal.ppat.0010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parkhill J, Sebaihia M, Preston A, et al. Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Nat Genet. 2003;35:32–40. doi: 10.1038/ng1227. [DOI] [PubMed] [Google Scholar]
- 11.Wolfe DN, Kirimanjeswara GS, Harvill ET. Clearance of Bordetella parapertussis from the lower respiratory tract requires humoral and cellular immunity. Infect Immun. 2005;73:6508–13. doi: 10.1128/IAI.73.10.6508-6513.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mann PB, Wolfe D, Latz E, et al. Comparative toll-like receptor 4-mediated innate host defense to Bordetella infection. Infect Immun. 2005;73:8144–52. doi: 10.1128/IAI.73.12.8144-8152.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andreasen C, Powell DA, Carbonetti NH. Pertussis toxin stimulates IL-17 production in response to Bordetella pertussis infection in mice. PLoS One. 2009;4:e7079. doi: 10.1371/journal.pone.0007079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirimanjeswara GS, Mann PB, Harvill ET. Role of antibodies in immunity to Bordetella infections. Infect Immun. 2003;71:1719–24. doi: 10.1128/IAI.71.4.1719-1724.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heininger U, Cotter PA, Fescemyer HW, et al. Comparative phenotypic analysis of the Bordetella parapertussis isolate chosen for genomic sequencing. Infect Immun. 2002;70:3777–84. doi: 10.1128/IAI.70.7.3777-3784.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harvill ET, Cotter PA, Miller JF. Pregenomic comparative analysis between Bordetella bronchiseptica RB50 and Bordetella pertussis Tohama I in murine models of respiratory tract infection. Infect Immun. 1999;67:6109–18. doi: 10.1128/iai.67.11.6109-6118.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gueirard P, Ave P, Balazuc AM, et al. Bordetella bronchiseptica persists in the nasal cavities of mice and triggers early delivery of dendritic cells in the lymph nodes draining the lower and upper respiratory tract. Infect Immun. 2003;71:4137–43. doi: 10.1128/IAI.71.7.4137-4143.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tuomanen E, Weiss A. Characterization of two adhesins of Bordetella pertussis for human ciliated respiratory-epithelial cells. J Infect Dis. 1985;152:118–25. doi: 10.1093/infdis/152.1.118. [DOI] [PubMed] [Google Scholar]
- 19.Weiss AA, Hewlett EL, Myers GA, Falkow S. Genetic studies of the molecular basis of whooping cough. Dev Biol Stand. 1985;61:11–9. [PubMed] [Google Scholar]
- 20.Mattoo S, Foreman-Wykert AK, Cotter PA, Miller JF. Mechanisms of Bordetella pathogenesis. Front Biosci. 2001;6:E168–86. doi: 10.2741/mattoo. [DOI] [PubMed] [Google Scholar]
- 21.Tuomanen EI, Nedelman J, Hendley JO, Hewlett EL. Species specificity of Bordetella adherence to human and animal ciliated respiratory epithelial cells. Infect Immun. 1983;42:692–5. doi: 10.1128/iai.42.2.692-695.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X, Hester SE, Kennett MJ, et al. Interleukin-1 receptor signaling is required to overcome the effects of pertussis toxin and for efficient infection- or vaccination-induced immunity against Bordetella pertussis. Infect Immun. 2011;79:527–41. doi: 10.1128/IAI.00590-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolfe DN, Mann PB, Buboltz AM, Harvill ET. Delayed role of tumor necrosis factor- alpha in overcoming the effects of pertussis toxin. J Infect Dis. 2007;196:1228–36. doi: 10.1086/521303. [DOI] [PubMed] [Google Scholar]
- 24.Pilione MR, Harvill ET. The Bordetella bronchiseptica type III secretion system inhibits gamma interferon production that is required for efficient antibody-mediated bacterial clearance. Infect Immun. 2006;74:1043–9. doi: 10.1128/IAI.74.2.1043-1049.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Read AF, Taylor LH. The ecology of genetically diverse infections. Science. 2001;292:1099–102. doi: 10.1126/science.1059410. [DOI] [PubMed] [Google Scholar]
- 26.Lina G, Boutite F, Tristan A, et al. Bacterial competition for human nasal cavity colonization: role of staphylococcal agr alleles. Appl Environ Microbiol. 2003;69:18–23. doi: 10.1128/AEM.69.1.18-23.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lysenko ES, Ratner AJ, Nelson AL, Weiser JN. The role of innate immune responses in the outcome of interspecies competition for colonization of mucosal surfaces. PLoS Pathog. 2005;1:e1. doi: 10.1371/journal.ppat.0010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Griffin AS, West SA, Buckling A. Cooperation and competition in pathogenic bacteria. Nature. 2004;430:1024–7. doi: 10.1038/nature02744. [DOI] [PubMed] [Google Scholar]
- 29.Hood RD, Singh P, Hsu F, et al. A type VI secretion system of Pseudomonas aeruginosa targets a toxin to bacteria. Cell Host Microbe. 2010;7:25–37. doi: 10.1016/j.chom.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joo J, Gunny M, Cases M, et al. Bacteriophage-mediated competition in Bordetella bacteria. Proc Biol Sci. 2006;273:1843–8. doi: 10.1098/rspb.2006.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aoki SK, Pamma R, Hernday AD, et al. Contact-dependent inhibition of growth in Escherichia coli. Science. 2005;309:1245–8. doi: 10.1126/science.1115109. [DOI] [PubMed] [Google Scholar]
- 32.Stibitz S, Yang MS. Subcellular localization and immunological detection of proteins encoded by the vir locus of Bordetella pertussis. J Bacteriol. 1991;173:4288–96. doi: 10.1128/jb.173.14.4288-4296.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cotter PA, Miller JF. BvgAS-mediated signal transduction: analysis of phase-locked regulatory mutants of Bordetella bronchiseptica in a rabbit model. Infect Immun. 1994;62:3381–90. doi: 10.1128/iai.62.8.3381-3390.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pishko EJ, Kirimanjeswara GS, Pilione MR, et al. Antibody-mediated bacterial clearance from the lower respiratory tract of mice requires complement component C3. Eur J Immunol. 2004;34:184–93. doi: 10.1002/eji.200324234. [DOI] [PubMed] [Google Scholar]
- 35.Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–9. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 37.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–10. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 38.Schloss PD, Westcott SL, Ryabin T, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75:7537–41. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huson DH, Auch AF, Qi J, Schuster SC. MEGAN analysis of metagenomic data. Genome Res. 2007;17:377–86. doi: 10.1101/gr.5969107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mills KH. Immunity to Bordetella pertussis. Microbes Infect. 2001;3:655–77. doi: 10.1016/s1286-4579(01)01421-6. [DOI] [PubMed] [Google Scholar]
- 41.Russell AB, Hood RD, Bui NK, et al. Type VI secretion delivers bacteriolytic effectors to target cells. Nature. 2011;475:343–7. doi: 10.1038/nature10244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.MacIntyre DL, Miyata ST, Kitaoka M, Pukatzki S. The Vibrio cholerae type VI secretion system displays antimicrobial properties. Proc Natl Acad Sci. 2010;107:19520–4. doi: 10.1073/pnas.1012931107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murdoch SL, Trunk K, English G, et al. The opportunistic pathogen Serratia marcescens utilizes type VI secretion to target bacterial competitors. J Bacteriol. 2011;193:6057–69. doi: 10.1128/JB.05671-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Majeed H, Gillor O, Kerr B, Riley MA. Competitive interactions in Escherichia coli populations: the role of bacteriocins. ISME J. 2011;5:71–81. doi: 10.1038/ismej.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown SP, Inglis RF, Taddei F. Evolutionary ecology of microbial wars: within-host competition and (incidental) virulence. Evol Appl. 2009;2:32–9. doi: 10.1111/j.1752-4571.2008.00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weyrich LS, Rolin OY, Muse SJ, et al. A type VI secretion system encoding locus is required for Bordetella bronchiseptica immunomodulation and persistence in vivo. PLoS One. 2012;7:e45892. doi: 10.1371/journal.pone.0045892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baron S, editor. Normal flora. Medical microbiology. Galveston, TX: University of Texas Medical Branch at Galveston; 1996. http://www.ncbi.nlm.nih.gov/books/NBK7617/ Accessed 8 January 2012. [Google Scholar]
- 48.Turnbaugh P, Ley RE, Hamady M, et al. The human microbiome project. Nature. 2007;449:804–10. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.